Regulatory Updates on Duchenne MD and CF Drug Translarna Provided by PTC
by |
 PTC Therapeutics has provided global regulatory updates on its lead product candidate Translarna (ataluren) for treating nonsense mutation Duchenne muscular dystrophy (nmDMD) and nonsense mutation cystic fibrosis (nmCF).
PTC Therapeutics has provided global regulatory updates on its lead product candidate Translarna (ataluren) for treating nonsense mutation Duchenne muscular dystrophy (nmDMD) and nonsense mutation cystic fibrosis (nmCF).
According to the National Institutes of Health Genetics Home Reference, a nonsense mutation is a change in one DNA base pair where instead of substituting one amino acid for another, the altered DNA sequence prematurely signals the cell to stop building a protein, resulting in a shortened protein that may either function improperly, or not at all. Nonsense mutations are implicated in a variety of genetic disorders.
Translarna for Genetic Disorders
Licensed in the European Economic Area, and investigational in other jurisdictions, Translarna is a novel, orally administered small-molecule compound in clinical development for treating patients with genetic disorders like nmDMD and nmCF that are caused by nonsense mutations.
Ataluren has been granted conditional marketing authorization in the European Union under the trade name Translarna for treatment of nmDMD in ambulatory patients ages 5 and older, and is the first treatment approved for the underlying cause of DMD. It has been classified as an orphan medicinal product by the European Medicines Agency (EMA), and the U.S. Food and Drug Administration (FDA) has also granted Translarna orphan drug designation for treating both nmDMD and nmCF.
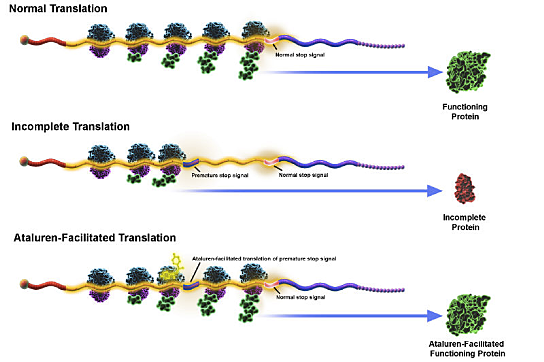
PTC Therapeutics believes Translarna interacts with the ribosome — the cellular component that decodes the messenger RNA (mRNA) molecule that carries genetic information from DNA to specify amino acid sequence — manufacturing proteins that enable the ribosome to read through premature nonsense stop signals on mRNA, allowing the cell to produce a full-length, functional protein.
The company says Translarna has the potential to become an important therapy for muscular dystrophy, cystic fibrosis, and other genetic disorders caused by nonsense mutations.
Graph courtesy PTC Therapeutics Inc.
European Zone Update on Translarna for nmDMD
PTC reports that over the past several months the company has been engaged in constructive discussions with the European Medicines Agency Committee for Medicinal Products for Human Use (CHMP) regarding renewal of Translarna’s marketing authorization, and has been informed that the necessary renewal assessment procedure cannot be completed by the middle of this year.
However, PTC says it expects that Translarna’s current provisional marketing authorization status will remain valid until a new decision is adopted by the European Commission, and that the CHMP has agreed to the company’s proposal to submit a draft clinical trial protocol for further discussion, which will include seeking scientific advice from the EMA.
PTC says it is optimistic that its undertaking to conduct the agreed-upon clinical trial will support renewal of Translarna’s current marketing authorization in the EC zone.
 “We appreciate all the effort put forth by the CHMP in this renewal process,” PTC Therapeutics CEO Stuart W. Peltz, PhD, said in a press release. “We are pleased that meanwhile patients will continue to have access to Translarna, the first-ever treatment approved for an underlying cause of Duchenne muscular dystrophy.”
“We appreciate all the effort put forth by the CHMP in this renewal process,” PTC Therapeutics CEO Stuart W. Peltz, PhD, said in a press release. “We are pleased that meanwhile patients will continue to have access to Translarna, the first-ever treatment approved for an underlying cause of Duchenne muscular dystrophy.”
Translarna’s initial provisional marketing authorization for the treatment of nmDMD in the European Economic Area was granted in 2014, subject to annual renewal and certain regulatory conditions. The drug, whose list price is approximately £220,000 (roughly $292,500) per year, is currently available to patients either commercially or through early access programs in more than 20 countries outside the U.S..
Phase 2 Clinical Study of Translarna for nmDMD in Pediatric Patients
As part of PTC’s commitments to facilitate Translarna’s provisional European marketing authorization and also to support potential expansion of Translarna’s label to younger patients, the company has launched a pediatric clinical trial investigating Translarna as a treatment of nmDMD in patients ages 2 to 5. The Phase 2, open-label, multiple-dose clinical trial will evaluate Translarna’s safety and pharmacokinetic efficacy in young pediatric patients.
UK National Institute for Health and Care Excellence (NICE) Translarna Guidance Update
PTC Therapeutics also reports that the U.K. National Institute for Health and Care Excellence (NICE) recently issued final guidance recommending Translarna for treatment of nmDMD patients in England in association with a National Health Services England (NHS) Managed Access Agreement (MAA) that waives the usual three-month funding period under local regulation, which could result in Translarna being available for treatment of nmDMD patients in England within weeks.
The successfully negotiated MAA authorizing Translarna treatment of ambulatory nmDMD patients ages 5 and older provides reimbursed patient access to Translarna in England for five years under a confidential financial agreement between NHS England and PTC. The company agrees to supply Translarna at a cost acceptable to the NHS, and the guidance will be reviewed again after five years. Translarna had previously received a positive recommendation from NICE in April 2016, subject to PTC and NHS England finalizing terms of the MAA.
Translarna for nmDMD in the US
PTC reports that it has recently participated in discussions with the FDA to discuss a Refuse to File (RTF) letter issued in February 2016 regarding the company’s New Drug Application (NDA) for Translarna for treatment of nmDMD. The company says it recently submitted an appeal to shift ongoing discussions regarding the RTF decision to the next FDA management level via the FDA Center for Drug Evaluation and Research (CDER)’s formal dispute resolution process. During the process, PTC affirms it is willing to consider multiple paths to advance potential FDA approval of Translarna, including conducting an additional clinical trial under FDA accelerated approval.
PTC to Host Conference Call to Discuss Second Quarter Financial Results
PTC will host a webcast conference call to report its second quarter financial results and provide an update on the company’s business and outlook at 4:30 p.m. Eastern time on Thursday, Aug. 4. The call can be accessed by dialing (877) 303-9216 (domestic) or (973) 935-8152 (international) five minutes before the start of the call and providing the passcode 49796148. A live, listen-only webcast of the conference call can be accessed on the investor relations section of the PTC website at https://www.ptcbio.com.
A webcast replay of the call will be available approximately two hours after completion of the call and will be archived on the company’s website for two weeks.