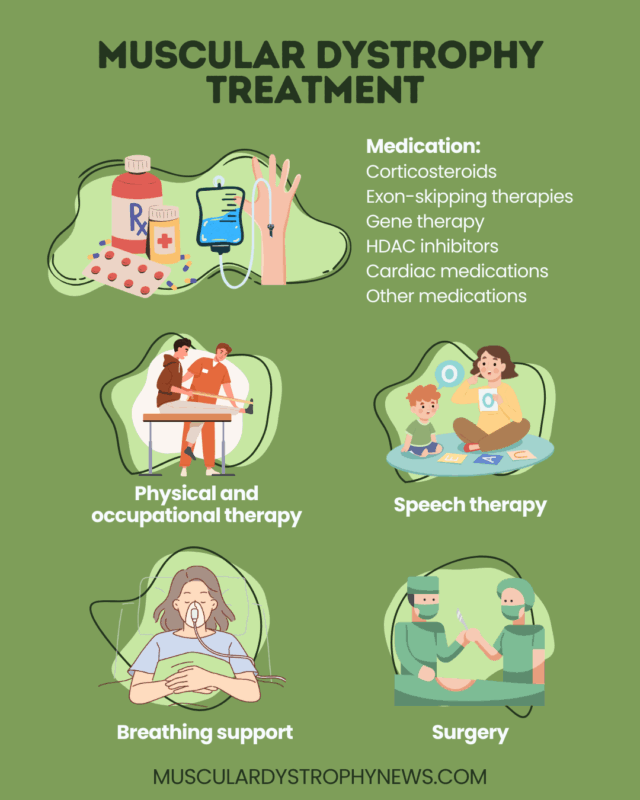
Muscular dystrophy treatment
Last updated Sept. 5, 2025, by Lindsey Shapiro, PhD

While there is no cure for muscular dystrophy (MD), the right treatment approach can ease symptoms and improve life quality for people living with these rare genetic muscular disorders.
There are more than 30 types of MD, which all share common features of progressive muscle weakness and wasting due to genetic mutations that affect muscle health.
MD treatment may involve medications, surgeries, and rehabilitation strategies to preserve function, prevent complications, and improve quality of life. For people with Duchenne muscular dystrophy (DMD), there are also approved therapies that can slow disease progression and extend MD life expectancy.
Medications for muscular dystrophy
There are a variety of medications that may be used as treatment for MD, depending on the disease type, underlying genetic mutation, and specific MD symptoms.
Corticosteroids
Oral corticosteroids work to ease the inflammation that contributes to muscle harm in MD, in turn preserving muscles and slowing the progression of muscle weakness and other complications.
These medications are the foundation of DMD treatment, and are usually started early and taken for life. They may also be used as a Becker MD treatment or for other MD types when muscle damage is prominent.
Corticosteroids for MD include:
- prednisone, which is not specifically approved for MD, but is routinely used off-label
- Emflaza (deflazacort), approved in the U.S. for DMD patients, ages 2 and older
- Agamree (vamorolone), approved in the U.S. for DMD patients, ages 2 and older
Long-term use of corticosteroids such as prednisone and Emflaza can lead to a range of potentially serious side effects, including weight gain, behavioral changes, weak bones, growth and puberty delays, high blood pressure, and others. Agamree is a dissociative steroid that’s designed to have the same therapeutic benefit, but with a more favorable safety profile.
Exon skipping therapies
Among the approved treatments for MD are exon-skipping therapies, which are designed to slow DMD progression by increasing the body’s production of dystrophin, a protein important for muscle health, which people with DMD make too little of due to mutations in the DMD gene. The therapies skip over certain problematic parts of the DMD genetic code to allow a shorter, but functional version of dystrophin to be made.
Exon-skipping therapies are given via weekly intravenous infusions into the bloodstream, and can be used by patients of all ages. Each is intended to treat patients with specific mutations:
- Amondys 45 (casimersen), for mutations amenable to exon 45 skipping
- Exondys 51 (eteplirsen), for mutations amenable to exon 51 skipping
- Viltepso (viltolarsen), for mutations amenable to exon 53 skipping
- Vyondys 53 (golodirsen), for mutations amenable to exon 53 skipping
Gene therapy
Gene therapy for MD is intended to slow or stop disease progression by directly addressing the disease’s underlying genetic cause.
Elevidys (delandistrogene moxeparvovec-rokl) is the only gene therapy approved in the U.S., where it can be used for DMD patients, ages 4 and older, who can or cannot walk. Given via a one-time intravenous infusion, Elevidys delivers a version of DMD to muscle cells, enabling the long-term production of a short, but functional version of dystrophin.
Several other gene therapies are in clinical development for DMD and other forms of MD.
Histone deacetylase inhibitors
Histone deacetylase (HDAC) inhibitors block enzymes belonging to the HDAC class, which are involved in cellular processes that regulate genetic activity. This strategy increases the production of follistatin, a protein that helps build muscle mass, and counteracts the effects of myostatin in preventing muscle growth and repair.
Duvyzat (givinostat) is the only HDAC inhibitor approved in the U.S. for DMD. Taken orally, Duvyzat can be used in adults and children, ages 6 and older.
Cardiac medications
Heart problems are a feature of several forms of MD, and cardiac medications may be needed to delay their onset or slow their progression.
- Angiotensin-converting enzyme (ACE) inhibitors lower blood pressure and make it easier for the heart to pump blood. Examples include lisinopril and enalapril.
- Angiotensin receptor blockers also target the renin-angiotensin system and may be used when ACE inhibitors aren’t tolerated. Examples include candesartan and losartan.
- Beta-blockers slow heart rate to allow the heart to work more efficiently. Examples include metoprolol, carvedilol, and propranolol.
- Mineralocorticoid antagonists lower blood pressure and can help delay heart scarring. Examples include spironolactone and eplerenone.
- Anti-arrhythmic medications work to normalize the heart’s rhythms. Examples include mexiletine, flecainide, and amiodarone.
Because virtually all people with DMD develop heart disease, it is recommended that heart medications be started by age 10, even if cardiac tests are still normal.
Cardiac medications for other forms of MD depend on the disease type and severity. For example, myotonic dystrophy treatment may involve interventions for managing arrhythmias, or heart rhythm abnormalities, as does the management of Emery-Dreifuss MD.
Other medications
Various other MD medications may be used as needed to control symptoms. These include:
- medications to improve bone health, such as biphosphonates, teriparatide, testosterone, or calcium supplements
- interventions for pain management
- treatments to ease gastrointestinal symptoms, such as stool softeners or antacids
Physical and occupational therapy
Beyond medications, there are non-drug interventions that are used to preserve muscle strength and make daily life easier for people with MD.
- MD physical therapy involves the use of appropriate exercise to help preserve muscle strength, prolong mobility, and manage pain.
- Occupational therapy focuses on helping people make daily life adaptations that enable them to be as mobile and independent as possible.
- Speech therapy uses exercises to improve speech clarity, communication, and swallowing function when muscles in the face, neck, and throat are affected.
These therapies for MD are guided by trained experts who will make adjustments based on a person’s individual needs. They’ll also guide patients in using assistive devices and home adaptations that can improve their quality of life as functional independence is lost.
Respiratory and cardiac support
As respiratory muscles weaken, people with MD may experience breathing problems and difficulty coughing, which can put them at risk for respiratory infections like pneumonia.
Monitoring and treating these issues is central to MD care. Respiratory support for MD may involve:
- breathing exercises
- cough assist devices or vest therapy, to help clear the airways when coughing is difficult
- ventilation assistance, or devices that help carry air in and out of the lungs
Regular cardiac monitoring is also key in MD. Where medications are not sufficient, patients may also require implanted devices to normalize heartbeat or help the heart pump blood.
Surgery
Surgery is sometimes needed to manage the complications of muscular dystrophy as the disease progresses. Surgical procedures used in MD include:
- spinal fusion surgery to correct scoliosis, an abnormal curvature of the spine that can affect mobility and breathing
- tendon release procedures to ease contractures, or the hardening and shortening of muscles and tendons that can lead to stiff and painful joints
- implantation of devices to manage heart rhythm abnormalities or help the heart pump blood, including pacemakers, cardioverter-defibrillators, or ventricular assist devices
- surgical removal of cataracts, a clouding of the eye’s lens that can impair vision
- breathing or feeding tube placement, if swallowing and breathing issues become severe
Whether and when surgery is needed will depend on the individual case, balancing the clinical need with any potential risks.
Experimental treatments for muscular dystrophy
Numerous experimental treatments are being investigated for MD. Some are designed to directly address the specific genetic causes of the disease, while others aim to ease inflammation, promote muscle repair, or preserve heart function through different mechanisms.
Given the genetic basis for MD, gene therapies are of substantial interest as they provide a new, healthy gene to replace the mutated version whose defect underlies the disease. While much research is focused on DMD, gene therapy is also being evaluated for other MD types, including as a possible limb-girdle MD treatment. Gene editing approaches that remove, add, or change sections of DNA have also been tested.
Lifestyle changes and complementary treatments
Managing MD may involve lifestyle changes and complementary therapies after an MD diagnosis. While they won’t directly alter the course of the disease, they can help make living with muscular dystrophy easier.
These could include:
- eating a healthy and balanced diet, along with nutritional supplements as recommended by a dietitian
- complementary therapies to ease pain and stiffness, such as acupuncture, massage therapy, or aquatic therapy
- using assistive devices or home adaptations to make it easier to get around
- staying up to date on recommended vaccines, to avoid respiratory infections
- finding ways to boost emotional well-being and mental health, such as support groups, counseling or therapy, and other self-care activities
Patients should discuss any major lifestyle changes with their care team before initiating them, to make sure they are safe in their own individual cases.
Muscular Dystrophy News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- MDA 2026: Keynote speaker to MDA community: ‘Your voice is powerful’
- Guest Voice: Navigating the windy road of rare disease specialists
- MDA 2026: More dietary protein linked to better lower limb function in MD
- ‘The Wonder Years’ were the ‘worry years’ before my MD diagnosis
- What’s on my wish list for my family’s Duchenne dream home