In DMD Patients, Translarna Works by Bypassing ‘Stop’ Sign to Create Functional Protein
Written by |
Translarna (ataluren) is a promising drug for the treatment of Duchenne muscular dystrophy (DMD) and cystic fibrosis. A team of scientists at University of Massachusetts Medical School and the University of Alabama at Birmingham has provided additional knowledge into Translarna’s mechanism of action, advancing our understanding of how the drug works to help patients.
The study, “Ataluren stimulates ribosomal selection of near-cognate tRNAs to promote nonsense suppression,” was published in the journal Proceedings of the National Academy of Sciences (PNAS).
The mechanism responsible for DMD is the occurrence of single-base pair mutations in DNA (a mutation affecting only one of the nucleotides in a gene). This error leads to a misplaced, premature “stop” codon in the middle of the gene.
This “stop sign” instructs the machinery of the cell to prematurely halt the production of the protein, leading to a defective protein that can no longer function. In DMD, the protein affected is called dystrophin, and its absence in muscle cells causes muscle tissue to be fragile and easily damaged.
Now, scientists discovered that Translarna seems to tell the cells’ machinery to bypass that stop sign and continue producing the total functional protein.
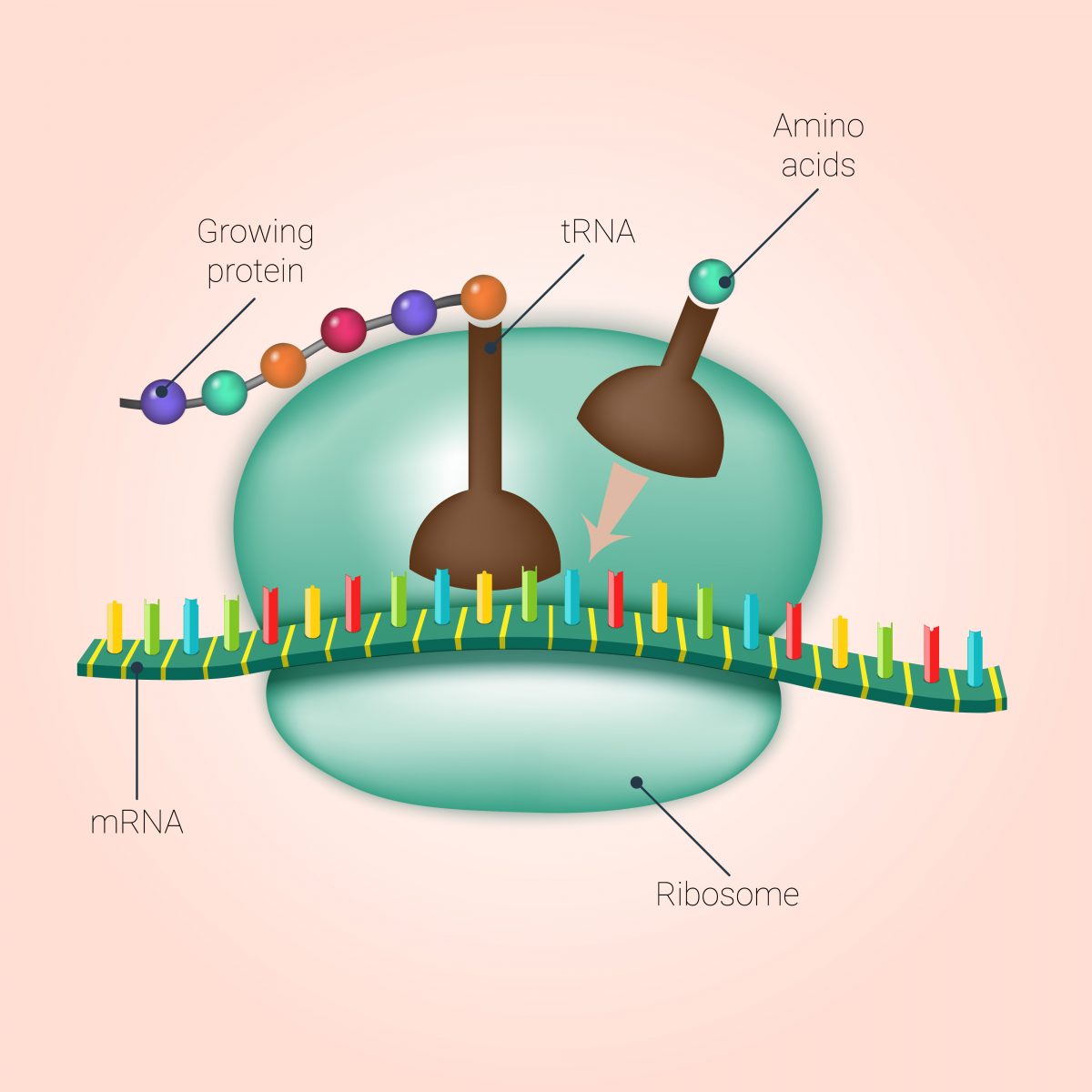
Allan Jacobson, PhD, of the University of Massachusetts Medical School and co-founder of PTC Therapeutics, the company that developed Translarna, and David Bedwell, PhD, professor of the University of Alabama Department of Biochemistry and Molecular Genetics, wanted to understand specifically how the drug instructed the ribosome, the place where protein synthesis occurs, to skip over these inserted stop signs and produce a functional protein.
Specifically, since amino acids are the building blocks of a protein, they wanted to know how Translarna modulates amino acid insertion. So researchers created numerous premature stop signs in test genes in human and yeast cells. They then analyzed what amino acids were inserted when Translarna allowed a skip-over of the mutation leading to the stop-sign premature insertion.
They found that Translarna acts at the ribosome and leads to the insertion of an amino acid that is similar to the one present in the non-mutated gene. This means that, although there was a mutation in the sequence of the DNA, Translarna actually overcomes this mistake by leading the ribosome to include an amino acid similar to the one of the healthy protein. Moreover, researchers found that the proteins produced with Translarna treatment were full-length, and retained other aspects of normal protein synthesis.
“These results further support our clinical findings demonstrating the production of full-length functional protein in nonsense mutation Duchenne muscular dystrophy and cystic fibrosis,” Stuart W. Peltz, PhD, co-founder and CEO of PTC Therapeutics, said in a press release. “Given this mechanism, ataluren offers the potential for a new therapeutic approach for multiple nonsense mutation genetic disorders by targeting the underlying cause of the disease.”
“Our new data is scientifically important because ataluren restores activity to genes inactivated by nonsense mutations, and as a result, it has the potential to do so much for a large number of very complex genetic disorders,” Jacobson concluded.





