Mouse Study Addresses Possibility of Enhancing DMD Respiratory Capacity Via Brain
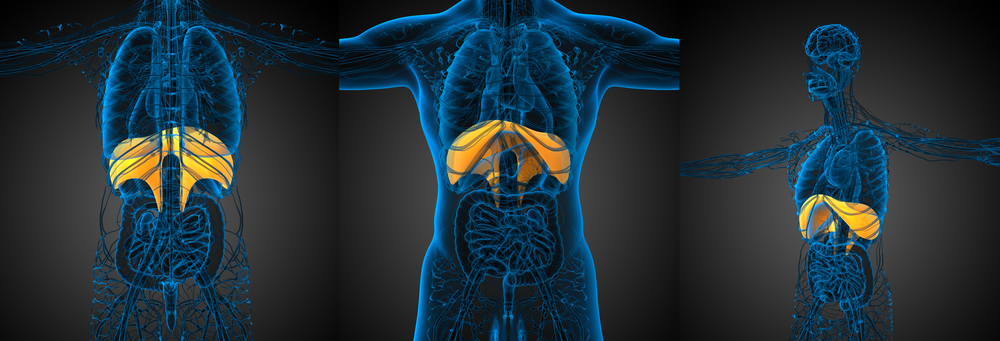
Respiratory activity is significantly impaired in Duchenne muscular dystrophy (DMD), but a new mouse study suggests that enhancing breathing via the brain may improve respiratory capacity.
The study “Sensorimotor control of breathing in the mdx mouse model of Duchenne muscular dystrophy” was published in The Journal of Physiology.
Respiratory failure is a leading cause of death in DMD, but there is a general lack of knowledge of the mechanisms regulating breathing in patients, or even in mouse models, of the disease.
To investigate this further, the research team of Ken O’Halloran at University College Cork, Ireland collaborated with researchers at the University of Calgary, Canada, and Trinity College Dublin, Ireland and performed experiments using a mouse model for DMD that lacked dystrophin, termed “mdx” mice.
They observed that the young (8 weeks of age) dystrophin-deficient mice had several impairments in their respiratory system, particularly in the diaphragm.
“Our study revealed profound muscle weakness and muscle fibre remodelling in young mdx diaphragm, suggesting severe mechanical disadvantage in mdx mice at an early age,” authors wrote.
The diaphragm is a skeletal muscle that extends across the bottom of the thoracic cavity and separates the thoracic and abdominal cavities, playing a key role in respiration. When we inhale, the diaphragm contracts, during which it is drawn into the abdominal cavity until it is flat. During exhale, the rib cage drops to its resting position while the diaphragm relaxes and returns to its dome-shaped position in the thorax.
The mice were anesthetized and scientists studied if brain activity could somehow influence the mice respiratory capacity. They examined the respiratory neural (related to nerves) drive to the diaphragm in baseline conditions (control) and in response to stimulation with low oxygen (hypoxia), hypercapnia (too much carbon dioxide in the blood) and asphyxia (severely deficient supply of oxygen).
The researchers performed these experiments in the dystrophin-deficient mice and in control (wild-type) mice and measured their diaphragms’ electrical activity by electromyography (EMG).
“Baseline diaphragm EMG activity was equivalent in wild-type and mdx mice,” researchers wrote, but the different stimulations enhanced diaphragm EMG activity in mdx mice.
These results suggest that in dystrophin-deficient mice, the brain may increase activation of the diaphragm muscle, working harder as a compensatory mechanism.
“Further study of physiological neuromuscular mechanisms that compensate for the absence of dystrophin is needed. A better understanding may provide insight with potential applications to a range of neuromuscular diseases, beyond DMD,” David Burns, PhD, researcher at University College Cork and study first author said in a press release.






