FDA gives WVE-N531, exon 53 skipping therapy, supportive status
Potential DMD therapy in ongoing Phase 1b/2a trial with early findings due soon
Written by |

The U.S. Food and Drug Administration (FDA) has given WVE-N531, an investigational therapy for Duchenne muscular dystrophy (DMD) patients amenable to exon 53 skipping, a rare pediatric drug designation.
This status aims to incentivize companies developing treatments for serious or life-threatening conditions that primarily affect children and are considered rare, defined in the U.S. as affecting fewer than 200,000 residents. If WVE-N531 goes on to be approved, Wave Life Sciences may qualify for an FDA voucher that can be used for priority review of a different therapy, or be sold to another company.
WVE-N531 is being evaluated in the open-label Phase 1b/2a FORWARD-53 trial (NCT04906460) that enrolled 11 male patients, ages 5 to 18, at sites in Jordan and the U.K. Results in treatment effects, including dystrophin levels from muscle tissue samples collected after 24 weeks of treatment (about six months), are expected before the end of September.
Exon-skipping therapies aim for a shorter but working dystrophin protein
“This designation from [the] FDA underscores that significant unmet needs remain in DMD,” Anne-Marie Li-Kwai-Cheung, Wave Life’s chief development officer, said in a company press release. “With our WVE-N531 program, we are aiming to restore clinically meaningful levels of near full length, functional dystrophin protein.”
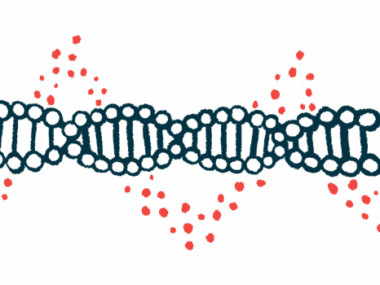
DMD is caused by mutations in the DMD gene that result in a lack of functional dystrophin, a protein important to protect muscle cells from damage, leading to disease symptoms like muscle weakness and wasting.
Like other genes, DMD is composed of several individual sections, called exons, which contain the information to produce dystrophin. When the gene is read, the exons are lined up and processed one after the other to produce the protein. Many Duchenne mutations cause the exons to be out of alignment, so the cell is not able to produce dystrophin.
Exon skipping is a DMD treatment approach to increase the levels of functional dystrophin in muscle cells by allowing the protein-producing cellular machinery to skip one or more exons when the gene is read, so the remaining exons fit together. This leads to the production of a shortened but functional version of dystrophin.
Specifically, WVE-N531 is designed to skip exon 53 in the DMD gene. About 8-10% of all DMD patients are amenable to skipping this exon, according to Wave.
FORWARD-53 is a two-part trial evaluating the infusion treatment’s safety, pharmacological properties, and clinical effects. Its 11 boys and teenagers are on stable corticosteroid therapy and have a DMD-causing mutation amenable for exon 53 skipping.
In part A, three ambulatory patients were treated with a single 1 mg/kg dose of WVE-N531, followed by single doses at 3, 6, and 10 mg/kg. After a safety evaluation, they were given three 10 mg/kg doses every other week.
Preliminary results from muscle biopsies taken two weeks after the final dose showed a mean of 53% exon skipping at the RNA level. RNA is a molecule produced as an intermediary step when a gene is processed to make a protein.
Dystrophin levels being assessed after 24 weeks of twice monthly treatment
The company also reports that WVE-N531 achieved a mean level in muscle tissue of about 42,000 nanograms/g, which it states to be 20-30 times higher than levels reported for other exon-skipping technologies.
WVE-N531 also was detected in patients’ myogenic stem cells, which are the precursors of myoblasts, the cells that give rise to functional muscle cells, Wave reported, noting WVE-N531 is the first therapy to be found in myogenic stem cells.
In the trial’s part B, all patients are being treated with 10 mg/kg doses every other week for 48 weeks (almost one year), with dystrophin levels determined in muscle tissue samples at 26 and 50 weeks.
Positive FORWARD-53 findings would “unlock additional programs for other exons in our pipeline, with the goal of developing best-in-class medicines that address the underlying cause of the disease for up to 40% of boys with DMD,” Li-Kwai-Cheung said.
In a preclinical study in nonhuman primates, WVE-N531 was detected in the heart and diaphragm — a dome-shaped muscle that is key for breathing — at higher levels than in skeletal muscle, the body’s most common type of muscle, indicating that it also may offer cardiac and respiratory benefits.
The open-label trial is due to conclude in May 2025.