Duchenne Muscular Dystrophy New Drug Application Submitted to the FDA for Translarna
 PTC Therapeutics, Inc. submitted a New Drug Application (NDA) to the United States Food and Drug Administration (FDA) for Translarna™. The medication is used for treating nonsense mutation Duchenne muscular dystrophy (nmDMD).
PTC Therapeutics, Inc. submitted a New Drug Application (NDA) to the United States Food and Drug Administration (FDA) for Translarna™. The medication is used for treating nonsense mutation Duchenne muscular dystrophy (nmDMD).
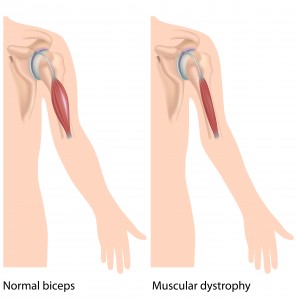
Duchenne muscular dystrophy (DMD) is a progressive disorder of the muscles that mostly affects males. It is caused by a lack of functional dystrophin, a protein that is important for stabilizing muscles in the diaphragm, heart and skeleton. People with severe DMD may no longer be able to walk even as early as childhood, and may also have life-threatening heart and lung problems in young adulthood. The nonsense mutation causes DMD about 13% of people with DMD (approximately 2,000 patients in the USA and 2,500 patients in the EU).
PTC is a biopharmaceutical company based in South Plainfield, NJ, that focuses on discovering small molecule oral medications for rare and orphan diseases. According to a press release issued by the company, a rolling submission has been filed. The release stated that “A rolling submission allows completed portions of the application to be submitted and reviewed by the FDA on an ongoing basis. PTC expects to finalize the application in the fourth quarter of 2015 following the completion of the ACT DMD confirmatory Phase 3 clinical trial.”
Translarna (also called ataluren) was discovered and developed by PTC Therapeutics, Inc. It restores the functioning dystrophin protein in people with the nonsense mutation. Translarna is already licensed in Europe for treating nonsense mutation Duchenne muscular dystrophy in patient who are able to walk and who are five years of age or older. Translarna is still under investigation the United States.
[adrotate group=”3″]
Several funding agencies have contributed grants to support the development of Translarna, including the Cystic Fibrosis Foundation Therapeutics Inc., the Muscular Dystrophy Association, the FDA’s Office of Orphan Products Development, the National Center for Research Resources; National Heart, Lung, and Blood Institute and Parent Project Muscular Dystrophy.
Stuart W. Peltz, Ph.D., Chief Executive Officer, PTC Therapeutics, Inc. stated “The initiation of our NDA submission for Translarna marks another significant milestone towards providing Translarna to all nonsense mutation Duchenne muscular dystrophy patients. We look forward to the completion of the ACT DMD confirmatory Phase 3 clinical trial so that we can finalize the NDA. Gaining US approval, in addition to Translarna’s European approval, will help to make Translarna available to patients across the globe. This is our commitment to the patients, families, advocacy groups and physicians who have worked and supported PTC Therapeutics through many years of research and development.”
“We want Translarna available for Duchenne patients in the US as fast as possible,” added Pat Furlong, President and Founder of PPMD. “We commend PTC’s perseverance and dedicated efforts to speed access to Translarna for people with Duchenne in the United States. Every day counts for people with Duchenne. Our hope is that PTC’s efforts pave the way for Translarna as well as other therapies in the US.”
Translarna is the first treatment that has been approved for the cause of DMD. In clinical trials the drug appears to delay the progression of muscle weakness.






