Dystrohin-Deficient Mice Resistant to Atherosclerosis
Experiments that produce unexpected results are often times more informative than predictable experiments. Such is the case for a new study that found an unexpected link between the protein dystrophin and the disease atherosclerosis. A research team from the Department of Experimental Medical Science at Lund University in Sweden discovered that a mouse model of Duchenne muscular dystrophy bearing a mutation in the mdx gene was resistant to atherosclerotic plaque buildup due to a deficiency in dystrophin production.
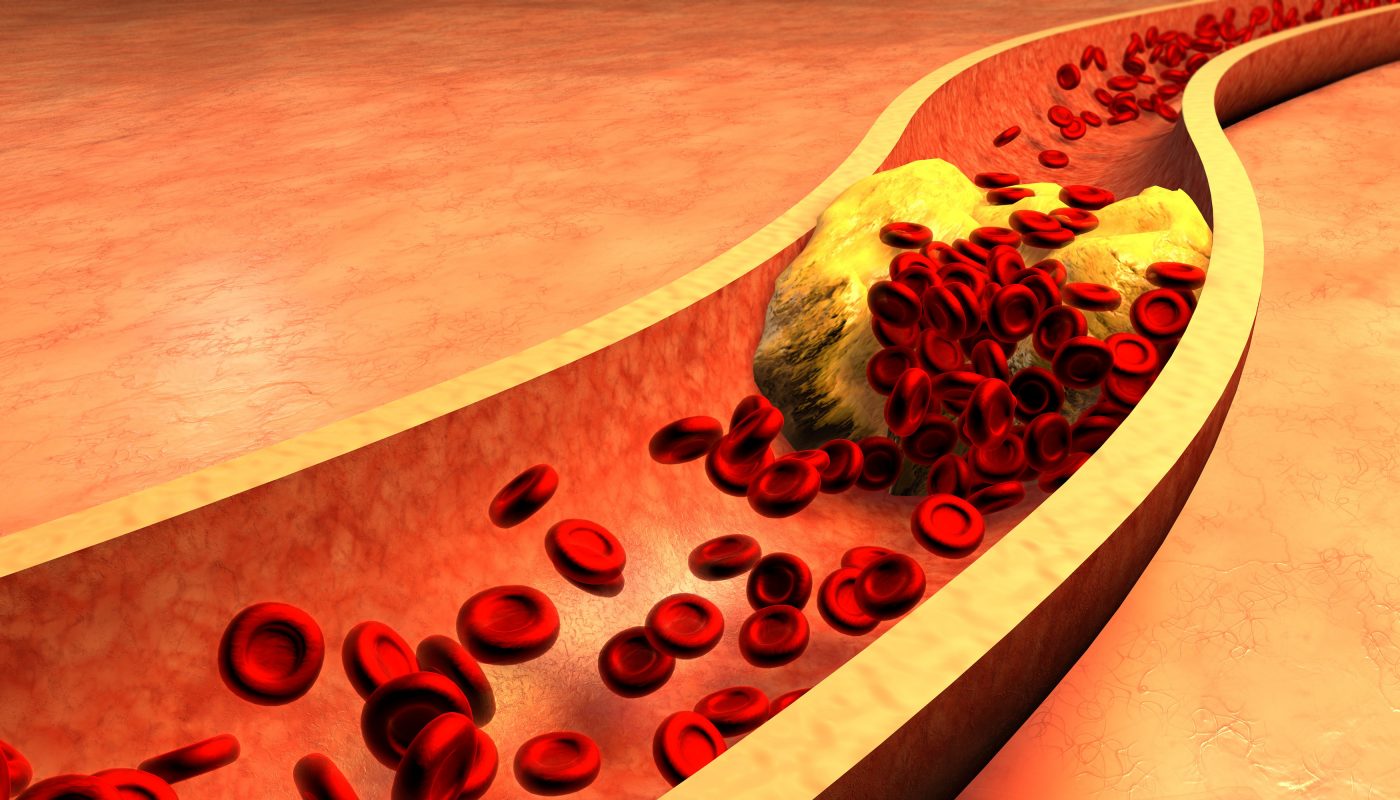
The most common cause of heart attack, atherosclerosis, is characterized by atherosclerotic plaque buildup in the walls of blood vessels. These plaques are composed of lipid-filled macrophages, and they form after lipids bind to proteins in the extracellular matrix between vascular smooth muscle cells (SMCs) and endothelial cells (ECs). The extracellular matrix is composed largely of the proteins laminin and collagen. The attachment of cells to the extracellular matrix is mediated by proteins on the surface of cells that bind to laminin and collagen. Among these proteins is dystrophin.
Since dystrophin serves as a connection between SMCs and arterial walls, Dr. Annelie Shami, lead author of the study, “Dystrophin Deficiency Redues Atherosclerotic Plaque Development in ApoE-null Mice,” published in Nature, hypothesized that inhibiting dystrophin could have an effect on atherosclerosis. Previous reports identified a decrease in dystrophin production by SMCs during injury to the vessels, suggesting that dystrophin deficiency may result in larger, more SMC-rich plaques in mice prone to developing atherosclerosis.
The team wanted to identify how cells connected to the extracellular matrix when dystrophin is present or absent. The researchers exposed carotid arteries from atherosclerosis-prone (ApoE-null) mice to shear stress and repeated the experiment in ApoE-null/mdx mutation mice. ApoE-null mice had smaller plaques develop than the ApoE-null/mdx mice following shear stress exposure. Sixty percent less lipid deposits were measured in ApoE/mdx mice.
As a means to identify why dystrophin deficiency prevented large plaques from growing, the researchers looked at other proteins in the extracellular matrix. In ApoE/mdx mice, there was less laminin protein production in plaques compared to ApoE mice. Therefore, since laminin and dystrophin were reduced, SMCs could not grow on the walls of blood vessels and contribute to artherosclerotic plaque formation.
Although the original hypothesis of the study was not supported, the results are informative about the role of dystrophin in artherosclerosis onset and progression. It is possible this connection is mediated through immune cells, but future studies would be necessary to confirm this hypothesis.






