BBP-418 for limb-girdle muscular dystrophy
Last updated Sept. 30, 2024, by José Lopes, PhD

What is BBP-418 for limb-girdle muscular dystrophy?
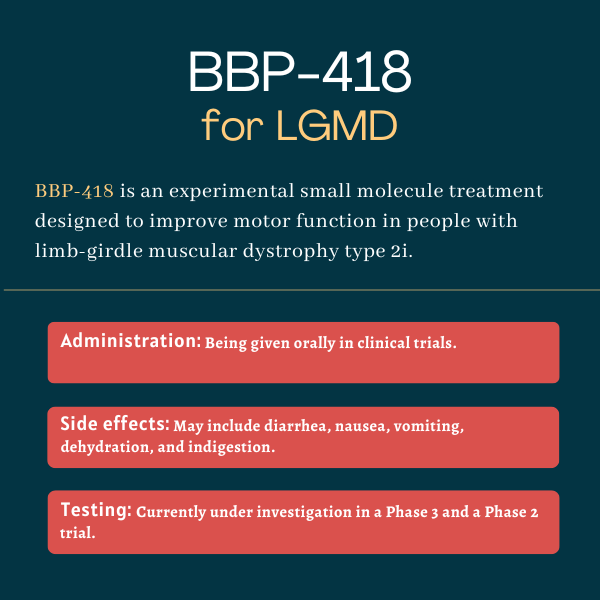
BBP-418, or ribitol, is an oral treatment candidate designed to improve motor function in people with limb-girdle muscular dystrophy (LGMD) type 2i.
The small molecule treatment is being developed by Bridgebio Pharma.
The U.S. Food and Drug Administration awarded BBP-418 rare pediatric disease, fast track, and orphan drug designations for LGMD2i. The European Medicines Agency also granted orphan drug designation to the therapy for LGMD. These designations are meant to support and accelerate the development of potential therapies for serious and/or rare conditions.
Therapy snapshot
| Treatment name: | BBP-418 |
| Administration: | Being tested as an oral formulation |
| Clinical testing: | Currently being tested in a Phase 3 and a Phase 2 trial |
How does BBP-418 work?
LGMD type 2i (LGMD2i), or LGMDR9, is a type of muscular dystrophy caused by mutations in the FKRP gene. FKRP provides instructions for the production of the fukutin-related protein (FRKP), which works to add specialized sugar molecules to alpha-dystroglycan — a process called glycosylation.
Alpha-dystroglycan is part of the dystrophin glycoprotein complex. This complex binds to the sarcoglycan protein complex and together they strengthen muscle fibers, stabilize the muscle cell membrane, and protect the muscle from contraction-induced damage. When the sarcoglycan complex cannot interact as intended with the dystrophin complex, muscles become more fragile and accumulate damage.
Mutations in FKRP lead to slowly progressive muscle weakness, especially in the hips and shoulders, with LGMD2i patients potentially experiencing issues such as a waddling gait, enlargement of the calves and tongue, heart muscle disease, and respiratory problems.
BBP-418 is designed to help increase alpha-dystroglycan glycosylation by enhancing the activity of the FKRP enzyme. It is a prodrug, or precursor molecule, which means that it gets converted into a sugar component of alpha-dystroglycan inside the body. Ultimately, the therapy is expected to improve motor function in LGMD2i.
How will BBP-418 be administered in LGMD?
In an ongoing Phase 3 clinical trial, BBP-418 has been given orally two times a day at 9 g or 12 g, depending on the patient’s body weight. Similar daily regimens (with adjustments for lower-weight participants) are also being tested in a Phase 2 trial. BBP-418 granules are reconstituted in water for oral administration.
However, it is too early to know which dose will be used if and when BBP-418 is approved by regulatory authorities.
BBP-418 in clinical trials
BBP-418 is being tested in a Phase 3 clinical trial called FORTIFY (NCT05775848) involving adolescents and adults, ages 12 to 60, with LGMD2i. A Phase 2 trial (NCT04800874) in people with LGMD2i, ages 12 to 55, is also taking place. A Phase 1 study in healthy volunteers was conducted previously.
Phase 1 trial
BBP-418’s safety and pharmacological properties were assessed in a Phase 1 clinical study that involved 93 healthy volunteers. Both single (maximum dose 15 g) and multiple dose regimens (1.5 g daily to 9 g twice daily) were tested and compared with a placebo.
Results showed the therapy was generally well tolerated. There were no study discontinuations or serious side effects reported. Most of the adverse events deemed probably or possibly related to the therapy were digestion-related, such as nausea. Pharmacological data showed no difference in exposure to the medication when taken with or without food.
Phase 2 trial
A Phase 2 trial is currently testing BBP-418 in 14 participants, ages 12 to 55. The study, sponsored by Bridgebio’s affiliate ML Bio Solutions, aims to determine the safety and tolerability of increasing doses of BBP-418. Adverse events are monitored for up to five years. Measures of the treatment’s pharmacological profile are part of the secondary goals of the trial.
Study design includes three groups, all receiving BBP-418. Doses given during the first three months range from 6 g once daily to 12 g twice per day. Over the next three months, participants receive the maximum dose of 12 g twice daily if they weigh more than 70 kg (154 lbs), or lesser doses (6 g or 9 g) if their weight is lower. An open-label extension of two years then follows, with doses of 9 g or 12 g twice daily depending on patients’ weight.
Participants have moderate to severe disease, defined as being able to complete the 10-meter walk test in up to 12 seconds unaided or as being unable to complete this test, respectively. All had taken part in a natural history study (NCT04202627) intended to track disease progression in the absence of treatment and to validate a muscle biomarker for LGMD2i.
As for safety, early results showed no serious side effects related to the treatment. At 15 months, three severe adverse events due to underlying disease were recorded, deemed unrelated to the treatment. Overall, the 14 adverse events deemed possibly or probably related to the therapy were generally mild digestive issues, including diarrhea. No patient discontinued the trial.
Regarding efficacy after 90 days (three months) of treatment, all three dosing regimens of BBP-418 led to significantly higher ratios of glycosylated alpha-dystroglycan, by 43% on average. A marker of muscle damage called creatine kinase was reduced with the treatment.
BBP-418 therapy improved the walking ability of LGMD2i patients, as seen with all three doses on the 10-meter walk test. This benefit contrasted with a decline in the same patients during the natural history study.
All these benefits were sustained at the 15-month time point. Creatine kinase levels had decreased by more than 75% in those receiving BBP-418.
Phase 3 trial
The FORTIFY study is measuring the long-term safety and efficacy of BBP-418 in adolescents and adults with LGMD2i. An estimated 81 participants are expected, who will be randomly assigned to receive BBP-418 twice daily at 9 or 12 g doses, depending on body weight, or a placebo.
The trial’s primary goals include assessing the frequency and severity of adverse events related to treatment, as well as changes in the North Star Assessment for LGMD, a tool for assessing LGMD patients’ functional motor abilities. Both analyses will be conducted up to 36 months of therapy, or about three years.
Secondary outcomes include measures of walking, lung function, and motor performance in the upper limbs. Changes in glycosylated alpha-dystroglycan in muscle will also be determined.
Bridgebio plans to use an early analysis of glycosylated alpha-dystroglycan levels at one year of treatment as a surrogate study endpoint to support the therapy’s accelerated approval. Top-line data from that analysis are expected in 2025.
Common side effects of BBP-418
In a Phase 2 clinical trial, side effects deemed possibly or probably related to treatment included diarrhea, nausea, vomiting, dehydration, indigestion, intestinal infection, and headaches.
Muscular Dystrophy News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
Related articles