Out-of-Step Muscle Cells Launch Fibrosis Development In Duchenne Muscular Dystrophy Patients
A new study published October 13 in The Journal of Cell Biology reveals that like a marching band falling out of step, muscle cells in Duchenne muscular dystrophy patients fail to perform in unison, and this breakdown of muscular synchronicity leads to proliferation of stiff fibrotic tissue within muscles.
Duchenne muscular dystrophy is a genetic disorder affecting around 1 in 3,600 boys. According to Wikipedia, it is caused by a mutation in the dystrophin gene, the largest gene located on the human X chromosome, which codes for the protein dystrophin, an important structural component within muscle tissue that provides structural stability to the dystroglycan complex (DGC) of the cell membrane. While both sexes can carry the mutation, females rarely exhibit signs of the disease. Children with this condition usually don’t show symptoms right away. Symptoms usually appear in male children before age 6 and may be visible in early infancy, but their muscles become progressively weaker over time as normal tissue is replaced by rigid connective tissue — a process known as fibrosis. It’s been unclear whether fibrosis causes the failure of muscle repair or regeneration, or rather failure of regeneration leads to fibrosis.
To find answers to this chicken-or-egg conundrum, researchers at the Children’s National Medical Center in Washington, DC, analyzed muscle biopsies from patients at different stages of several dystrophic diseases and found that they expressed a collection of genes associated with both normal tissue repair and progressive fibrosis. They then looked at the expression of these genes in normally regenerating mouse muscle and found that, although the same genes were expressed, their activities were ordered and spread out over time in the healthy tissue, with every cell arriving at the same stage of the repair process alongside its neighbors. In contrast, cells in dystrophic muscle underwent these processes asynchronously, which could cause regeneration failure.
In order to test this hypothesis, the researchers created a mouse model of asynchronous muscle repair by injecting a toxin into nearby spots of muscle at different time intervals. When they analyzed the gene expression patterns over a period of 10 days, they found that muscle recovery stalled in areas of tissue between the two injury sites.
The JCB paper entitled: “Asynchronous remodeling is a driver of failed regeneration in Duchenne muscular dystrophy (JCB vol. 207 no. 1 139-158 The Rockefeller University Press, doi: 10.1083 jcb.201402079) is coauthored by Eric P. Hoffman, Sherry Dadgar, Zuyi Wang, Helen Johnston, Akanchha Kesari, Kanneboyina Nagaraju, Yi-Wen Chen, D. Ashley Hill, Terence A. Partridge, Mamta Giri, Robert J. Freishtat, and Javad Nazarian of the Center for Genetic Medicine Research, Childrens National Medical Center, and Department of Integrative Systems Biology at George Washington University, Washington; and Jianhua Xuan and Yue Wang of The Bradley Department of Electrical and Computer Engineering, Virginia Polytechnic Institute and State University, Arlington, Virginia. S. Dadgar, Z. Wang, and H. Johnston contributed equally to this paper.
[adrotate group=”3″]
The researchers sought to determine the mechanisms underlying failure of muscle regeneration that is observed in dystrophic muscle through hypothesis generation using muscle profiling data (human dystrophy and murine regeneration). They report finding that transforming growth factor centered networks strongly associated with pathological fibrosis and failed regeneration were also induced during normal regeneration, but at distinct time points.
They hypothesized that asynchronously regenerating microenvironments are an underlying driver of fibrosis and failed regeneration, and validated this hypothesis using an experimental model of focal asynchronous bouts of muscle regeneration in wild-type (WT) mice. A chronic inflammatory state and reduced mitochondrial oxidative capacity were observed in bouts separated by 4 d, whereas a chronic profibrotic state was seen in bouts separated by 10 d. Treatment of asynchronously remodeling WT muscle with either prednisone or VBP15 mitigated the molecular phenotype.
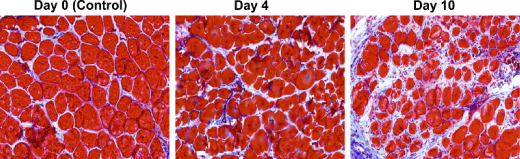
 Image Caption: This time series shows the proliferation of stiff connective tissue, or fibrosis, in a section of mouse muscle located between two distinct sites undergoing asynchronous regeneration. – Image Credit: Dadgar et al., 2014
Image Caption: This time series shows the proliferation of stiff connective tissue, or fibrosis, in a section of mouse muscle located between two distinct sites undergoing asynchronous regeneration. – Image Credit: Dadgar et al., 2014
The coauthors conclude that their asynchronous regeneration model for pathological fibrosis and muscle wasting in the muscular dystrophies is likely generalizable to tissue failure in chronic inflammatory states in other regenerative tissues.
 “The areas between the injections got frozen or fixed in a developmental time frame and had trouble getting out of it,” explains the paper’s senior author Eric Hoffman in a Rockefeller University Press release. “Because they were getting mixed signals from their neighbors as to where they should be in this regenerative process, they couldn’t do the right thing.”
“The areas between the injections got frozen or fixed in a developmental time frame and had trouble getting out of it,” explains the paper’s senior author Eric Hoffman in a Rockefeller University Press release. “Because they were getting mixed signals from their neighbors as to where they should be in this regenerative process, they couldn’t do the right thing.”
Rather than progressing through normal recovery and regeneration, the muscle tissue was being replaced by fibrotic tissue. These results therefore suggest that asynchronous regeneration of cells within muscle tissue leads to the development of fibrosis in patients with Duchenne muscular dystrophy. Dr. Hoffman’s group is working to develop steroids that can resynchronize regenerative processes with the hope that such drugs can be used to treat or prevent fibrosis in muscle and other tissues, such as the heart, lung, and liver.
A world-renowned human geneticist, Eric Hoffman, PhD, also co-leads the Systems Biology Initiative of the Sheikh Zayed Institute for Pediatric Surgical Innovation. The Systems Biology team integrates biological sciences, computational sciences, and engineering to map the complex interactions of biological activity with greater precision than ever before. Additionally, Dr. Hoffman directs the Research Center for Genetic Medicine at Children’s National and is the James Clark Chair in Molecular Genetics as well as chair of the Department of Integrative Systems Biology at the George Washington University.
The center is an interdisciplinary research facility of 100 scientists working on the most common childhood disorders as well as advanced therapeutic approaches for specific rare disorders. The center integrates state-of-the-art genome-enabled approaches with drug development and clinical trial networks. Dr. Hoffman has more than 400 publications and is among the more frequently cited scientists working today. His center receives about $10 million a year in research support of projects related to muscular dystrophy, asthma, obesity, type 2 diabetes, sports medicine, and brain disorders. Dr. Hoffman received his doctorate in biology genetics from Johns Hopkins University and completed post-doctoral studies at Harvard Medical School in Boston, Massachusetts.
Sources:
The Rockefeller University Press
The Journal of Cell Biology
Children’s National Medical Center
Wikipedia
Image Credits:
Children’s National Medical Center
Dadgar et al.






