Elevidys for Duchenne muscular dystrophy
What is Elevidys for Duchenne muscular dystrophy?
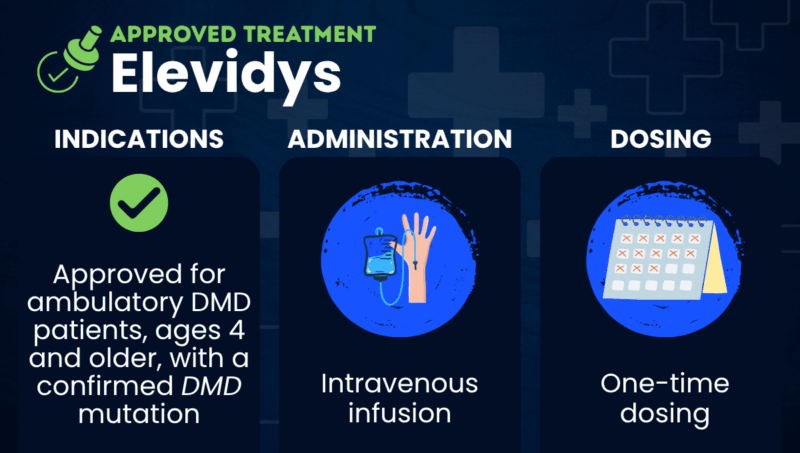
Elevidys (delandistrogene moxeparvovec-rokl), previously known as SRP-9001, is a one-time gene therapy approved in the U.S. to treat patients with Duchenne muscular dystrophy (DMD) ages 4 and older who are still able to walk (ambulatory).
DMD is caused by mutations in the DMD gene that result in little to no production of dystrophin, a protein important for muscle health. Muscles consequently become progressively damaged and weak over time.
Elevidys is designed to deliver to the body a gene encoding a shortened, but still functional dystrophin protein called micro-dystrophin. This should help protect muscle cells from damage and improve motor function.
The therapy is designed to be most active in heart and skeletal muscle cells — the tissues most affected in DMD. It is packaged into a viral carrier called AAVrh74 that helps it be taken up by muscle cells when administered as a single infusion into the bloodstream (intravenous infusion).
Elevidys is marketed by its developer Sarepta Therapeutics in the U.S. and by Roche in other countries.
Therapy snapshot
| Brand name | Elevidys |
| Chemical name | Delandistrogene moxeparvovec-rokl |
| Usage | Used to treat ambulatory DMD patients |
| Administration | Intravenous infusion |
Who can take Elevidys?
Elevidys is approved in the U.S. for the treatment of ambulatory DMD patients, ages 4 and older, who have a confirmed DMD gene mutation.
It is contraindicated, or should not be used by patients who have deletion mutations affecting all or part of exon 8 and/or exon 9 in the DMD gene. Exons are the coding sections of a gene that have the information needed for cells to produce proteins.
Elevidys comes with a boxed warning that it could cause serious liver injury, including life-threatening or fatal acute liver failure. It is not recommended for patients with existing liver impairments or an active viral liver infection.
The gene therapy is also not recommended for people who have had a vaccination or infection within the preceding month. It is generally avoided in people with significant levels of preexisting antibodies against AAVrh74.
How is Elevidys administered?
Elevidys is administered by a healthcare provider as a single intravenous infusion that takes about one or two hours. The recommended dose is:
- 133 trillion vector genomes per kilogram of body weight (vg/kg), for patients weighing 10 to 70 kg (about 22-154 pounds)
- 9.31 quadrillion vg/kg, for patients weighing 70 kg or greater
Before treatment, patients will be evaluated to rule out significant anti-AAVrh74 antibodies, active or recent infections, and liver problems. Platelet counts and levels of troponin-I, a marker of heart damage, will also be measured.
Prior to treatment, patients will also be started on a course of corticosteroids to prevent immune reactions. Corticosteroid treatment should be started a day before the Elevidys infusion for patients already on steroids and a week before the infusion for those not currently taking these medications. These are usually continued for at least 60 days (about two months) and then slowly tapered off. The exact corticosteroid dosing will be selected based on the patient’s existing corticosteroid regimen and adjusted if liver abnormalities occur.
Patients will be monitored by healthcare providers for at least three hours after the infusion on the day of treatment and will receive weekly blood tests for the first few months to monitor for complications.
Elevidys in clinical trials
Elevidys’ approval was supported by data from two completed placebo-controlled clinical trials and an ongoing open-label study, which collectively involved 214 DMD patients.
- A placebo-controlled Phase 2 trial (NCT03769116) involved 41 DMD patients, ages 4-7. The results showed that Elevidys led to significantly greater improvements in motor abilities than a placebo after a year among the subset of patients ages 4-5, as assessed with the North Star Ambulatory Assessment (NSAA). Data from an open-label extension period showed that Elevidys was associated with motor gains relative to the expected course of the disease in untreated patients.
- A placebo-controlled Phase 3 trial called EMBARK (NCT05096221) involved 125 ambulatory boys with DMD, ages 4-7. While NSAA scores tended to improve with Elevidys relative to a placebo, the difference was not statistically significant after a year. Other measures of walking abilities also tended to improve with the gene therapy. Long-term data showed that these gains were maintained for up to two years.
- The ongoing, open-label Phase 1b ENDEAVOR trial (NCT04626674) involves 48 DMD patients of varying ages and walking abilities. Results from the subset of ambulatory participants, ages 4-7, demonstrated stabilized or improved motor function a year after treatment with Elevidys.
All of these studies generally showed that Elevidys led to increases in micro-dystrophin levels, demonstrating that the therapy was working as intended in the body.
Elevidys side effects
The most common side effects associated with Elevidys include:
- vomiting and nausea
- liver injury
- fever
- low blood platelet counts
- elevations in troponin-I
Serious liver injury most often occurs within two months of the Elevidys infusion. According to the boxed warning, the risk of these complications is higher in people with preexisting liver problems.
The therapy should not be administered to such patients until acute liver disease is resolved or controlled. Liver function will be assessed before the infusion and weekly for the first three months afterward. It will then be monitored until the results return to normal. Patients will receive corticosteroids before and after the Elevidys infusion to prevent liver complications.
Patients are advised to maintain proximity to an appropriate healthcare facility, as determined by their healthcare provider, for at least two months after the infusion. If acute serious liver injury or impending liver failure is suspected, patients should promptly consult a liver specialist.
Elevidys also comes with warnings that it could cause the following potentially serious side effects:
- serious or fatal infections
- serious or life-threatening inflammation of the heart muscle (myocarditis)
- infusion-related reactions, including life-threatening reactions involving the airways, that may require the infusion to be slowed, paused, or stopped
- immune-mediated muscle inflammation (myositis), which could be severe or life-threatening, particularly in people with certain DMD mutations
Patients will be counseled on the signs and symptoms of these complications, carefully monitored for them, and treated appropriately if they arise.
Muscular Dystrophy News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
 Fact-checked by
Fact-checked by 

