Duvyzat (givinostat) for Duchenne muscular dystrophy
Last updated April 4, 2024, by Margarida Maia, PhD

What is Duvyzat for Duchenne muscular dystrophy?
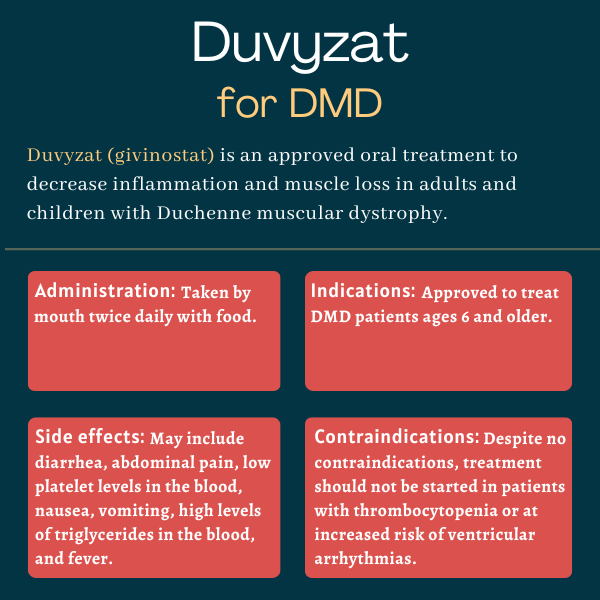
Duvyzat (givinostat) is an oral therapy approved in the U.S. for adults and children, ages 6 and older, with Duchenne muscular dystrophy (DMD). Developed by Italfarmaco, it aims to reduce inflammation and muscle loss in people with DMD, ultimately working to slow disease progression.
The small molecule treatment also is being developed for Becker muscular dystrophy (BMD), another type of muscular dystrophy. It was tested in a Phase 2 trial.
Therapy snapshot
| Brand name: | Duvyzat |
| Chemical name: | Givinostat |
| Usage: | Treatment for DMD patients 6 and older |
| Administration: | Given by mouth as an oral suspension |
How does Duvyzat work?
Both Duchenne and Becker are caused by mutations in the DMD gene, which provides instructions for producing the dystrophin protein. In DMD, no or little functional dystrophin is usually produced. Meanwhile, in BMD, some of the protein is produced, but it’s usually impaired or not sufficient to maintain muscle health.
Without sufficient dystrophin, patients’ muscles become more sensitive to damage from wear and tear, resulting in the progressive loss of muscle mass and strength that marks each disease type.
Duvyzat is a small molecule designed to block enzymes of the histone deacetylase (HDAC) family, which are involved in turning off gene activity.
Blocking HDAC enzymes can switch on the gene coding for a protein called follistatin. Follistatin helps to build muscle mass by counteracting the effects of myostatin, a protein that causes scar tissue to build up in muscles, preventing their growth and repair.
Treatment with Duvyzat is expected to ease muscle loss and inflammation in DMD, thereby slowing disease progression.
Who can use Duvyzat?
Duvyzat won U.S. regulatory approval in March 2024 for the treatment of adults and children, ages 6 and older, with DMD. Its use as a Duchenne therapy is under regulatory review in Europe, where the medication has been granted orphan drug designation.
Who should not use Duvyzat?
There are no contraindications for the use of Duvyzat, according to its prescribing information. However, treatment should not be started in patients with fewer than 150,000 platelets per microliter of blood. This is because Duvyzat can cause thrombocytopenia, or a decrease in the number of platelets in the blood. Platelets help the blood to clot, and having too few of them can increase the risk of bleeding or bruising.
Treatment with Duvyzat also should be avoided in patients who are at increased risk of ventricular arrhythmias, or irregular heartbeats that originate in the ventricles, which are the heart’s lower chambers.
How is Duvyzat administered?
Duvyzat comes as an oral suspension to be taken twice daily by mouth with food. The amount taken depends on body weight, with the lowest dose of 22.2 mg (2.5 mL) two times per day for patients weighing 10 kg to less than 20 kg (22 pounds to less than 44 pounds). The highest dose is 53.2 mg (6 mL) twice a day for those weighing 60 kg (132 pounds) or more.
The oral suspension is available at 8.86 milligrams per milliliter (mg/mL) in a bottle containing 140 mL of a white to off-white or faintly pink, peach-flavored liquid. Before use, the oral suspension should be shaken for at least 30 seconds by inverting the bottle by 180 degrees. The prescribed volume should be measured using the syringe that comes with Duvyzat.
The dose may need to be reduced if a patient:
- has less than 150,000 platelets per microliter of blood in two tests performed one week apart
- develops moderate or severe diarrhea
- has more than 300 mg of triglycerides (a type of fat) per deciliter of blood in two tests performed one week apart while fasting, which should cause the levels of triglycerides to decrease.
If side effects persist after adjusting the dose, it may need to be further reduced, depending on the patient’s body weight. If side effects persist after the second adjustment, treatment with Duvyzat should be stopped.
Duvyzat in clinical trials
Duvyzat was approved based on the results of a Phase 3 clinical trial testing its safety and efficacy in boys with DMD who were able to walk.
Clinical trials in DMD
An open-label, two-part Phase 1/2 clinical trial (NCT01761292) first tested the therapy’s safety and tolerability, as well as its pharmacokinetics, or its movement into, through, and out of the body. That study, which enrolled 20 boys with DMD, ages 7 to 10, who were able to walk, also evaluated the effects of Duvyzat on muscle tissue. All of the boys who participated were on a stable dose of corticosteroids.
After taking Duvyzat for one year or longer, the boys’ muscle fibers had grown larger by a mean of 78% and occupied 29% more area within muscle tissue. There also was less fat and scar tissue. While Duvyzat was not found to result in better muscle function, the researchers noted that was possibly due to the study’s sample size being too small to draw conclusions. Duvyzat was safe and well tolerated, with platelet count reductions being a dose-limiting side effect.
A pivotal Phase 3 clinical trial called EPIDYS (NCT02851797) then tested the safety and efficacy of Duvyzat against a placebo in 179 boys with DMD, ages 6 to 17, who were able to walk. All boys were on a stable dose of Emflaza (deflazacort) or other corticosteroids for six months or longer. The participants were randomly assigned to receive either Duvyzat (10 mg/mL) or the placebo twice daily, according to the child’s weight, for 18 months, or 1.5 years.
Duvyzat was shown to slow muscle function decline compared with baseline, or the start of the clinical trial. Boys on Duvyzat took an average of 1.78 fewer seconds to climb four stairs than those on the placebo. In that four-stair climb test, a shorter time is a better result.
Over 18 months of follow-up, Duvyzat also slowed a decline in the North Star Ambulatory Assessment score, a measure of motor function in which a higher score indicates better performance. Boys on Duvyzat scored an average 1.91 points higher compared with those on the placebo.
Moreover, boys on Duvyzat did not build up as much fat tissue in muscles as did those in the placebo group. For example, in a muscle in the thigh, the area occupied by fat tissue increased by 7.6% in boys on Duvyzat versus 10.6% in those on the placebo, a difference of 3%.
The most common side effects reported with Duvyzat were diarrhea, abdominal pain, thrombocytopenia, and high levels of triglycerides in the blood. Most (95%) were mild to moderate in severity, with three boys discontinuing Duvyzat due to a side effect.
Ongoing clinical trials
Boys who completed the 18-month clinical trial were invited to roll over into an ongoing extension study (NCT03373968). All those participating in this part will continue to receive Duvyzat at a starting dose equal to the one they were on at the end of the EPIDYS clinical trial. Side effects will be monitored for about one year.
Another Phase 3 clinical trial, dubbed ULYSSES (NCT05933057), will test the therapy in boys who have lost the ability to walk. ULYSSES will enroll as many as 138 boys with DMD, ages 9 to 17, who are not ambulatory. The study is planned to continue through 2028, and is expected to take place at multiple clinical sites in Europe and Canada.
As in EPIDYS, Duvyzat will be tested against a placebo and patients will be on a stable dose of corticosteroids. The primary outcome measure in ULYSSES is a change in the total score of Performance of Upper Limb 2.0, a tool to evaluate upper extremity function. Lung function, vital signs, and side effects also will be assessed.
Clinical trial in BMD
Further clinical testing also is anticipated for givinostat in Becker MD. A Phase 2 clinical trial (NCT03238235), completed in 2021, tested the safety, tolerability, and efficacy of givinostat against a placebo in 51 men with BMD. The participants ranging in from 18 to 65, were able to walk 200 to 450 meters (about 219 to 492 yards) in six minutes. All were randomly assigned to receive either givinostat or the placebo twice daily for 12 months (one year).
While the clinical trial failed to meet its main goal of reducing scarring after 12 months compared with the placebo, givinostat prevented fat tissue from building up in muscles. It also prevented the loss of muscle tissue.
Common side effects of Duvyzat
The most common side effects reported with Duvyzat in people with DMD are:
- diarrhea
- abdominal pain
- thrombocytopenia, or reduced numbers of platelets in the blood
- nausea
- vomiting
- high levels of triglycerides in the blood
- fever.
Thrombocytopenia
Duvyzat can cause dose-related myelosuppression, which occurs when the bone marrow becomes less active and produces fewer blood cells. This can result in thrombocytopenia, as well as anemia and neutropenia. Anemia is having too few red blood cells or too little hemoglobin to carry oxygen to the body’s tissues, while neutropenia means a person has too few neutrophils, or white blood cells.
Blood counts should be checked every two weeks for the first two months of treatment, then monthly for the first three months, and every three months thereafter. If thrombocytopenia develops, the dose of Duvyzat may need to be adjusted or treatment stopped.
Symptoms of thrombocytopenia include excessive bleeding from cuts, easy bruising, blood in stools or urine, and small red or purple spots on the skin called petechiae. If any of these symptoms are noticed, medical attention should be sought.
Increased triglycerides
Duvyzat can increase the levels of triglycerides in the blood, which can raise the risk of heart disease and stroke. Levels of triglycerides should be checked one, three, and six months after starting treatment with Duvyzat, and every six months thereafter. If triglycerides increase beyond 300 mg per deciliter of blood, the dose of Duvyzat may need to be adjusted or treatment stopped.
Gastrointestinal problems
Duvyzat can cause vomiting and diarrhea. If these occur, patients should drink plenty of fluids and keep track of the frequency and severity of their symptoms. The dose of Duvyzat may need to be adjusted, or treatment stopped if these side effects cannot be managed with antidiarrheal or antiemetic (against vomiting and nausea) medications.
Heart problems
Duvyzat can prolong the corrected QT (QTc) interval, a measure of the heart’s electrical activity on an electrocardiogram (ECG). This measure represents the time it takes the heart muscle to contract and then recover. A prolonged QTc interval indicates that the heart is taking longer than usual to recharge, which increases the risk of a form of an abnormal heart rhythm originating in the ventricles.
An ECG should be performed before starting Duvyzat in patients with underlying cardiac disease or in those taking medications that can cause QT prolongation. Treatment should be avoided in patients at increased risk of ventricular arrhythmias, such as those who have congenital long QT syndrome, coronary artery disease, or electrolyte disturbance, which occurs when the levels of certain minerals in the blood get too high or too low. It also should be avoided among those who use other medications known to prolong the QT interval.
Patients who experience fainting, dizziness, or loss of consciousness should inform their doctor. Treatment with Duvyzat should be stopped if the QTc interval is greater than 500 milliseconds (ms) or the change from baseline is greater than 60 ms.
Use in pregnancy and breastfeeding
DMD most commonly occurs in boys and men. Therefore, there is not enough data to advise on the use of Duvyzat in women who are pregnant or breastfeeding. However, studies in pregnant rats and rabbits showed that treatment with Duvyzat resulted in lower fetal weight, higher number of stillbirths, and neurobehavioral changes in the offspring. It is not known if Duvyzat passes into breast milk or if it is safe for a breastfed infant.
Muscular Dystrophy News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
Related articles