CureDuchenne Sets Calendar for Workshops, Sessions and ‘Futures’ Conference
Written by |
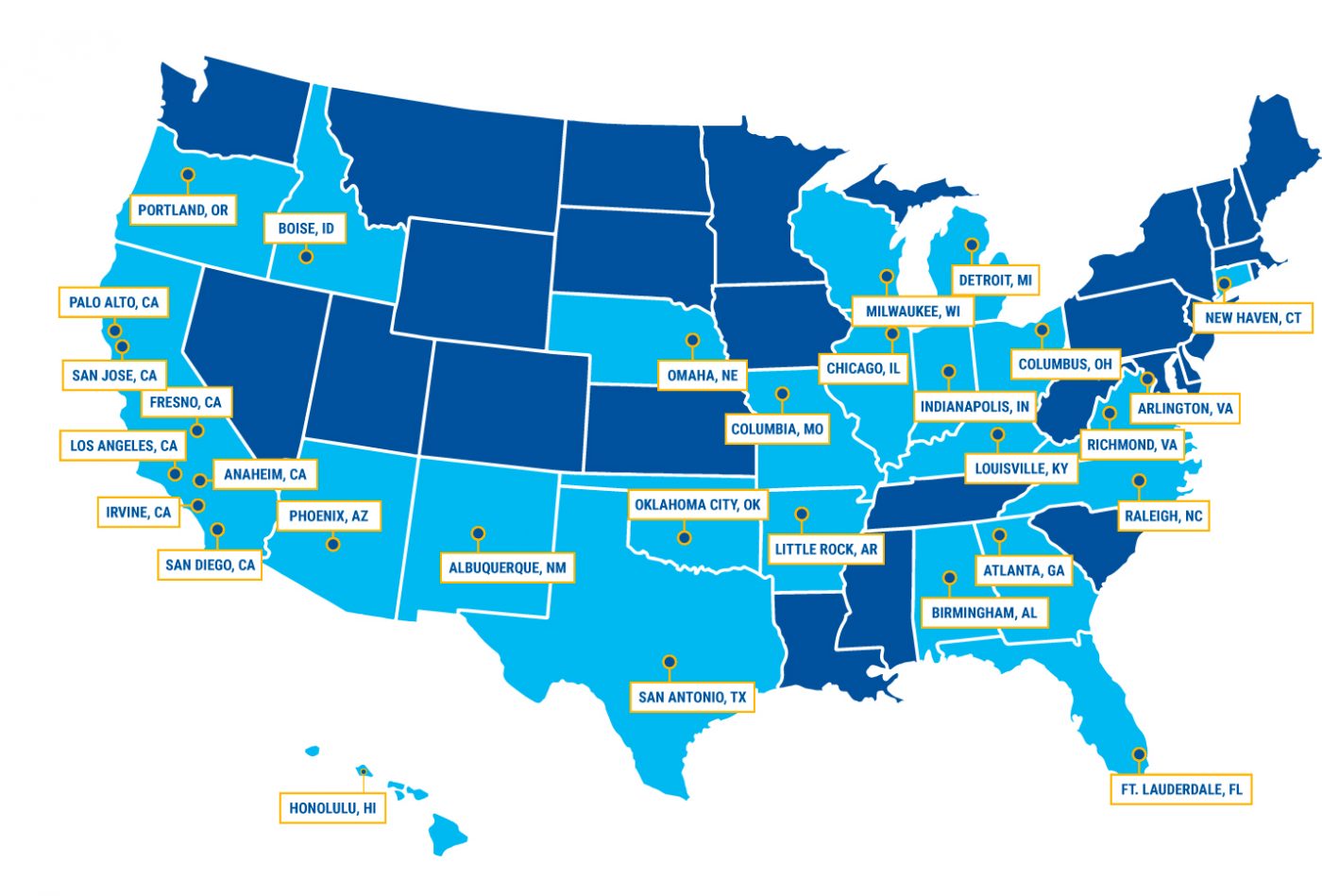
CureDuchenne plans 30 events across the nation in 2019.
CureDuchenne, one of the nation’s leading Duchenne muscular dystrophy (DMD) charities, is sponsoring 30 events across the U.S. this year to educate patients and their families about all aspects of the neuromuscular disease.
“Our motto is ‘leave no boy behind,’ so we want to make sure we have a format that works in smaller, less populated markets,” Debra Miller, CureDuchenne’s co-founder and CEO, told Muscular Dystrophy News in a phone interview.
To that end, 20 family and caregiver sessions have been scheduled under the CureDuchenne banner throughout the country. Upcoming ones include Los Angeles (March 23); Arlington, Virginia (March 30); Omaha, Nebraska (April 6); Phoenix (April 13); and San Diego (April 20).
“These are usually two- or three-hour lunch or dinner meetings, where we bring in a guest speaker to discuss topics not typically covered during clinic visits,” Miller said. “We’re able to talk to families and pull them together so they can connect with each other and interact in a casual way.”
The 2019 CureDuchenne calendar also includes nine one-day workshops, continuing a format established seven or eight years ago.
Workshops are full-day events in which clinicians, scientists and guest speakers share their wealth of Duchenne knowledge and discuss advancements in the field. Caregivers learn about physical therapy, get valuable updates about clinical trials, and learn about best practices in Duchenne care.
Interested in Muscular Dystrophy News research? Sign up for our forums and join the conversation!
In addition, CureDuchenne offers financial assistance to families traveling more than 100 miles, and on-site child care is available.
Upcoming workshops have been scheduled for March 16 in Columbia, Missouri; April 26-27 in Chicago, and May 4 in Albuquerque, New Mexico. Similar events are also planned for Detroit; Honolulu; Richmond, Virginia; and San Antonio, Texas.
A particularly important event is CureDuchenne’s Futures, scheduled for Oct. 11-13 at the Disneyland Hotel in Anaheim, California.
Miller said she expects 250 people to attend this year’s Futures conference, up from 100 who came to last year’s event, held Nov. 3-4 in Boston. Among the speakers will be two well-known names in Duchenne research: Brenda Wong, MD, who heads the Duchenne Program at UMass Memorial Medical Center in Worcester, Massachusetts, and Eric Olson, MD, director of the Hamon Center for Regenerative Science and Medicine at University of Texas-Southwestern Medical Center in Dallas.
Earlier this month, UT-Southwestern announced that Olson’s lab — using CRISPR gene editing techniques — may be able to treat Duchenne boys with an exon 44 deletion. This is the second most common deletion in Duchenne, accounting for 11 percent of all DMD cases (the most common is exon 51 deletion, comprising about 13 percent of patients).
“This is really good news,” Miller said. “Dr. Olson has shown incredible dystrophin restoration in both dog and mouse models of Duchenne. It’s looking hopeful that he’ll be able to treat different mutations with slightly different versions of his CRISPR.”
At the moment, three companies — Pfizer, Sarepta Therapeutics and Solid Biosciences — are pursuing human clinical trials for gene therapy to treat Duchenne.
CureDuchenne, headquartered in Newport Beach, California, has 16 employees and a 2019 budget of $7 million. Last year, the organization spent 81 percent of its $5 million budget on research and education.
The group’s portfolio currently includes 16 wide-ranging projects, which include establishing CureDuchenne Ventures in 2014. The organization says investments from Ventures have leveraged more than $1.3 billion in follow-on financing from venture capital, biotech and pharmaceutical companies to fund emerging projects toward treating DMD.





