FAQs about Elevidys
While there are no known interactions between Elevidys and alcohol, adult patients who receive the gene therapy are advised to speak with their healthcare team about whether it is safe to drink alcohol while on this medication.
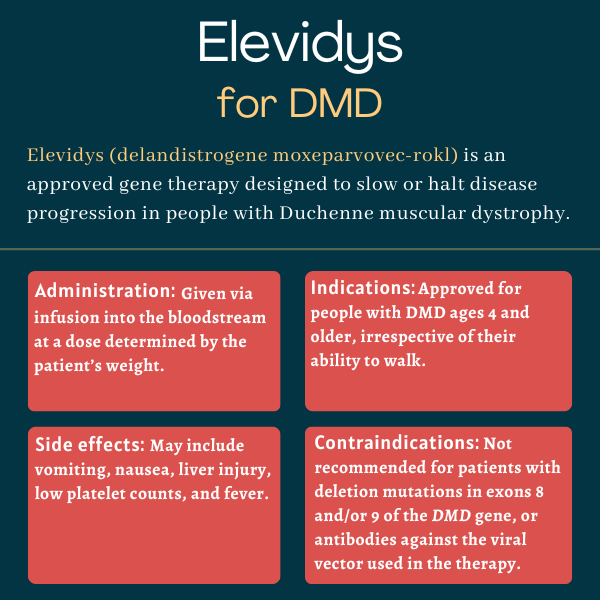
Duchenne muscular dystrophy (DMD) is characterized by lack of functional dystrophin, a protein that normally acts like a shock absorber in muscle cells, helping to cushion them and prevent wear and tear during movement. Elevidys provides a gene coding for a so-called micro-dystrophin protein, which is smaller but works similarly to normal dystrophin. The therapy is designed to have high affinity for muscle tissue, and greater gene activity in cardiac and skeletal muscles.
Results from a Phase 1/2 trial have shown improvements in motor function 90 days after treatment, namely in a rating scale called North Star Ambulatory Assessment (NSAA), time to rise, four-stair climb test, and the 100-meter timed test. Likewise, a Phase 2 trial showed micro-dystrophin production and NSAA score improvements at 12 weeks (around three months) of therapy. These benefits have been sustained in more recent assessments. There is, however, no standard timeline for Elevidys’ benefits, as each person may respond differently to the medication.
The most common side effects of Elevidys are vomiting, nausea, fever, low counts of platelets, and increased markers of liver damage. The therapy’s prescribing information also includes warnings about the risk of infusion reactions, serious liver injury, myositis, or muscle inflammation — particularly in patients with deletions in certain DMD exons — and myocarditis (inflammation of the heart muscle). Measurements of appropriate biomarkers and assessment of related symptoms are recommended before and periodically after starting treatment.
The U.S. Food and Drug Administration approved Elevidys in June 2023 to treat Duchenne muscular dystrophy (DMD) patients, ages 4 to 5, with a confirmed mutation in the DMD gene and who are able to walk. The decision, marking the first approval of a gene therapy for DMD, was granted under the agency’s accelerated approval pathway. The FDA expanded approval of Elevidys in 2024, granting the therapy traditional approval for ambulatory DMD patients ages 4 and up, as well as accelerated approval for nonambulatory patients in the same age range.
Related Articles

 Fact-checked by
Fact-checked by