Pamrevlumab for Duchenne muscular dystrophy
Last updated July 12, 2024, by Marisa Wexler, MS

What is pamrevlumab for Duchenne muscular dystrophy?
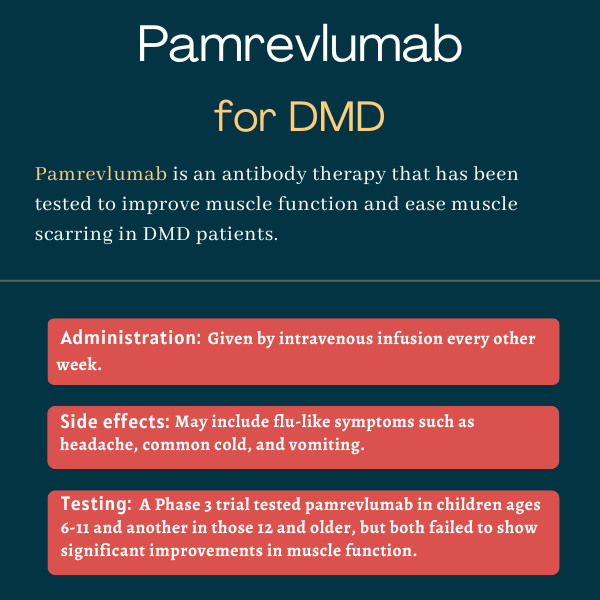
Pamrevlumab is an experimental infusion therapy that has been tested in people with Duchenne muscular dystrophy (DMD). Administered as an into-the-vein, or intravenous, infusion, it was designed to improve motor function and reduce muscle scarring.
However, it’s not clear if FibroGen is still developing the therapy for that indication, as two Phase 3 trials of pamrevlumab in DMD failed to meet their primary goals in 2023.
The company said it would be evaluating the totality of the data to determine next steps for pamrevlumab’s development for DMD, and so far, no further update has been announced.
FibroGen is now advancing development of pamrevlumab for certain forms of pancreatic cancer.
Therapy snapshot
| Treatment name: | Pamrevlumab |
| Administration: | Intravenous infusion |
| Clinical testing: | Completed two Phase 3 trials, both with negative results |
How does pamrevlumab work?
DMD is caused by mutations that result in a lack of the protein dystrophin. This protein normally acts like a “shock absorber” in muscles, and without it, muscles accumulate more wear-and-tear damage over time.
As muscles become increasingly damaged, the normal tissue gets replaced by scar tissue. This phenomenon, known as tissue fibrosis, reduces the ability of damaged muscle cells to repair, further contributing to the progression of disease symptoms such as muscle weakness.
Pamrevlumab is an antibody-based medication designed to reduce muscle fibrosis in DMD. It blocks the activity of a signaling molecule called connective tissue growth factor, which is an important driver of fibrosis and is found at elevated levels in DMD muscles.
How is pamrevlumab administered in DMD?
Pamrevlumab is administered via an infusion into the bloodstream. In DMD patients, two Phase 3 trials tested it at a dose of 35 mg per kilogram of body weight (mg/kg), given every other week.
However, as findings from those trials were negative, it’s unclear if the drug will continue to be developed for DMD, or if a new dose or dosing regimen will be tested in future DMD trials.
Pamrevlumab in clinical trials
After findings from a Phase 2 study called MISSION (NCT02606136) suggested pamrevlumab could slow muscle function decline in people with DMD compared with untreated historical controls, FibroGen launched a pair of Phase 3 clinical trials dubbed LELANTOS-1 (NCT04371666) and LELANTOS-2 (NCT04632940) to assess the therapy in different groups of DMD patients.
Phase 2 MISSION study
The open-label MISSION trial assessed the safety and effectiveness of pamrevlumab in 21 boys and young men, 12 and older, who had already lost the ability to walk independently.
All participants were given pamrevlumab (35 mg/kg) every two weeks for up to about two years, while continuing standard treatment with corticosteroids, namely prednisone or Emflaza (deflazacort).
The trial’s goals were to measure changes in respiratory function, as well as in arm strength, muscle strength, fat accumulation in muscle tissue, and fibrosis. Because MISSION did not include a placebo group, results from pamrevlumab-treated patients were compared to findings from a historical group of DMD patients who were given only standard corticosteroids.
Results from MISSION showed lung and heart function, as well as upper limb function, tended to worsen over time in patients on pamrevlumab. However, in some measures, the rate of worsening was significantly slower than what was seen in historical controls, implying that the therapy might be slowing disease progression. No serious side effects related to pamrevlumab were reported in the study.
After completing the main study, 15 patients enrolled in an open-label extension part, where all would continue to receive pamrevlumab for up to four more years. However, the extension study was terminated by the company in late 2023.
Phase 3 LELANTOS trials
To further test the efficacy of pamrevlumab in DMD, FibroGen conducted a pair of Phase 3 clinical trials dubbed LELANTOS.
While LELANTOS-1 enrolled in 99 DMD patients 12 and older who were not able to walk, LELANTOS-2 included 73 boys with DMD, ages 6 to 11, who still retained the ability to walk. Patients in both studies were randomly assigned to receive either pamrevlumab or a placebo, in addition to standard corticosteroid treatment.
The main goal of the study in nonambulatory patients was to see if pamrevlumab could outperform the placebo at improving a measure of arm function called the performance of upper limb (PUL) 2.0. The study in ambulatory patients used a measure of motor function designed for DMD boys who can walk called the North Star Ambulatory Assessment (NSAA).
Both studies failed to meet their main goals, with PUL 2.0 and NSAA scores showing no significant difference between patients given pamrevlumab or the placebo. The study in ambulatory patients also showed no significant difference in measures such as the ability to climb stairs or time to loss of walking ability.
Common side effects of pamrevlumab
Available safety findings from the Phase 3 trials of pamrevlumab in DMD indicated the therapy was well tolerated in both studies, with most treatment-related side effects being mild or moderate. Detailed safety data from these studies have not yet been announced.
No serious side effects deemed related to the medication were reported in the Phase 2 MISSION study. The most common side effects linked to pamrevlumab were flu-like symptoms such as:
- headache
- common cold
- vomiting
- cough
- fever.
Muscular Dystrophy News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Accepting help is difficult for me as a mom and a caregiver
- Precision’s gene-editing therapy gets FDA’s rare pediatric disease status
- An odd dream made me wonder how my disability affects others
- FDA advisory committee meeting for deramiocel in DMD set for July
- Finding balance while parenting children with DMD can be tricky
Related articles