Agamree (vamorolone) for Duchenne muscular dystrophy
What is Agamree for Duchenne muscular dystrophy?
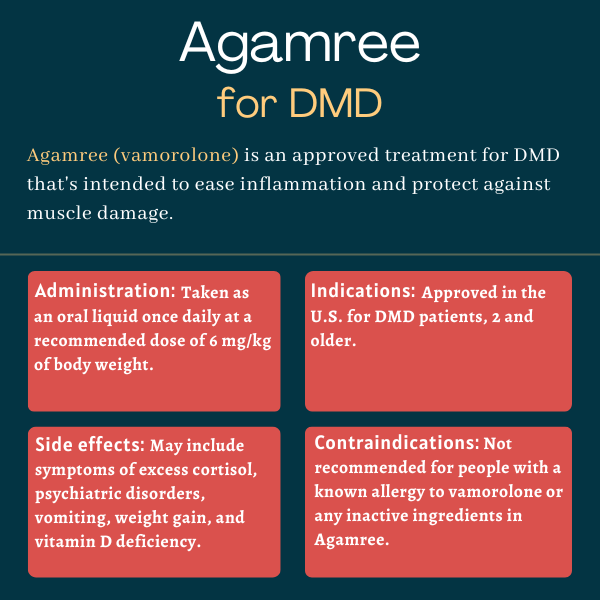
Agamree (vamorolone) is a so-called dissociative steroid for preserving muscle function and easing inflammation in people with Duchenne muscular dystrophy (DMD), ages 2 and older.
Catalyst Pharmaceuticals obtained the North American marketing rights to Agamree from Santhera Pharmaceuticals. The therapy was originally developed by ReveraGen BioPharma.
Therapy snapshot
| Brand name: | Agamree |
| Chemical name: | Vamorolone |
| Usage: | Therapy for Duchenne Muscular Dystrophy |
| Administration: | Oral liquid suspension |
How does Agamree work?
In Duchenne, mutations in the DMD gene cause no production of the dystrophin protein that’s important for protecting muscle cells when they contract. As a result, patients have symptoms of progressive muscle damage and wasting. Chronic inflammation contributes to the progression of this damage over time.
Corticosteroids, which mimic the effects of the hormone cortisol, are a class of potent anti-inflammatory medications commonly prescribed in DMD — e.g. prednisone, Emflaza (deflazacort). They’re thought to help preserve muscle tissue by easing inflammation, but are associated with a wide range of side effects when used long term. Agamree is a new type of corticosteroid called a dissociative corticosteroid that is intended to maximize the benefits of corticosteroids while minimizing their side effects.
Cortisol and corticosteroids exert their effects by interacting with cortisol receptors, leading to the activation of a wide range of cellular pathways. Some of these pathways lead to anti-inflammatory effects, while others can contribute to unwanted side effects. Agamree interacts with cortisol receptors in a way that promotes anti-inflammatory pathways, but avoids ones linked to traditional corticosteroids’ side effects.
For example, Agamree, like other corticosteroids, inhibits NF-kB pathways, which are involved in driving inflammation. It also helps stabilize cell membranes while preventing transactivation, an increase in gene activity seen with other corticosteroids that’s thought to contribute to side effects. Agamree may also help preserve heart function in DMD by blocking activity at proteins called mineralocorticoid receptors, which is not observed with other medications in this class.
Who can use Agamree?
Agamree was approved by the U.S. Food and Drug Administration in October 2023 for treating DMD patients ages 2 and older.
The therapy has also been approved in the European Union (plus Iceland, Liechtenstein, and Norway) and the U.K. for DMD patients ages 4 and older.
Who should not use Agamree?
Agamree should not be used by people with a history of allergic reaction’s to its active ingredient, vamorolone, or any of its inactive ingredients.
How is Agamree administered?
Agamree comes as an orange-flavored liquid suspension, white to off-white in color, at a concentration of 40 mg/mL. It should be taken by mouth once daily, preferably with a meal.
The recommended daily dose is 6 mg/kg of body weight up to a maximum daily dosage of 300 mg for people weighing more than 50 kg (about 110 pounds). The dose can be titrated down to as low as 2 mg/kg daily based on a person’s clinical response and tolerability. This lower dose is also recommended for patients with mild or moderate liver impairments.
Patients can be switched from standard oral corticosteroids, such as prednisone or Emflaza, without treatment interruptions or a need to first gradually lower the dose of the earlier medication. Patients switching from long-term treatment with another oral corticosteroid should start at a daily dosage of 6 mg/kg daily.
The Agamree oral suspension should be shaken well for about 30 seconds before being administered. The appropriate dose should be withdrawn using only the provided oral syringe and dispensed directly into the mouth. The shelf-life of Agamree is three months. Any open medication should be discarded after that time.
When stopping Agamree, the dose should be decreased gradually for any patient who has been taking it for more than one week.
Agamree in clinical trials
After establishing the safety of Agamree in a Phase 1 trial (NCT02415439) clinical trial involving healthy men, Agamree was tested in a Phase 2a trial (NCT02760264) involving 48 boys with DMD, ages 4 to 6. The participants were assigned to one of four oral doses of the therapy (0.25, 0.75, 2.0, or 6.0 mg/kg per day) daily for two weeks.
All four dose levels were well tolerated, and biomarker analyses indicated Agamree reduced inflammation while having an enhanced safety profile relative to traditional corticosteroids, including reduced suppression of the adrenal glands that produce cortisol, signs of improved bone health, and less insulin resistance, a feature of diabetes.
Participants continued at their assigned dose for 24 more weeks (about six months) in an extension study (NCT02760277). Results from this part showed Agamree led to dose-dependent improvements in performance on the time-to-stand test of muscle strength (TTSTAND) after 12 and 24 weeks, meeting the primary efficacy outcome. Participants also saw dose-dependent improvements in other functional measures. Safety and biomarker analyses were generally consistent with earlier trial analyses.
The participants then continued at their assigned dose in another 24-month extension trial (NCT03038399). Published results demonstrated Agamree at its highest two doses was associated with maintained muscle strength and function up to 30 months, and had similar efficacy to external groups of DMD patients receiving standard-of-care corticosteroids. It was also associated with improved growth relative to standard glucocorticoids, where growth delay was observed.
VISION-DMD trial
Applications for Agamree’s regulatory approval were largely backed by data from the VISION-DMD trial (NCT03439670), which enrolled 121 boys with DMD who could walk, ages 4-6. The participants were assigned to receive oral Agamree (2 or 6 mg/kg), prednisone (0.75 mg/kg), or a placebo once daily for six months. All were then given Agamree for another six months.
Agamree led to significant improvements in TTSTAND scores compared to a placebo after six months, meeting the trial’s main goal. Agamree also outperformed a placebo in a number of other secondary outcomes related to motor function. These gains were comparable to those seen with prednisone, but Agamree was associated with fewer side effects. While the boys on prednisone experienced growth delay, this was not observed with Agamree.
After nearly a year, patients who had switched from prednisone to Agamree did not see a loss in treatment efficacy. After the switch, these patients also saw a 30% reduction in side effects and a 60% reduction in side effects linked to corticosteroids, including a reversal of stunted growth, decreased behavioral changes, and stabilized body weight.
Ongoing trials
A Phase 2 trial (NCT05166109) is testing Agamree’s safety, effectiveness, and pharmacological properties against a placebo in men with Becker muscular dystrophy, ages 18-64, at a single site in Pittsburgh. The study is expected to finish in 2025.
Side effects of Agamree
The most common side effects associated with Agamree include:
- cushingoid features, or symptoms linked to excess cortisol
- psychiatric disorders
- vomiting
- weight gain
- vitamin D deficiency.
Altered endocrine function
Corticosteroids such as Agamree can cause serious and possibly life-threatening changes in the function of the body’s hormone-secreting (endocrine) glands, especially with chronic use.
This may include symptoms of Cushing’s syndrome, a condition where cortisol in the body is too high, or increased blood sugar levels, raising the risk of new or worsening diabetes. Patients using Agamree should be monitored for these conditions.
When stopping Agamree, patients should be monitored for adrenal insufficiency, where the adrenal glands don’t produce enough cortisol. The risk of this occurring is reduced by gradually tapering the dose when stopping treatment. In some cases, particularly when there are high levels of stress during treatment discontinuation, supplementation with another corticosteroid may be recommended.
A withdrawal syndrome may also occur when abruptly stopping treatment that is not related to adrenal insufficiency, but rather is attributed to sudden changes in corticosteroid concentrations. Symptoms may include anorexia, nausea, vomiting, lethargy, headache, fever, joint pain, skin peeling, muscle pain, or weight loss.
People with certain existing endocrine conditions, including disorders of the adrenal, pituitary, and thyroid glands, may be at an increased risk of adverse endocrine events. Patients with these conditions should always talk with their doctors about the benefits and risks of using Agamree in their particular case. Adjusting certain medications used to treat existing endocrine disorders, or of Agamree, may be required when used at the same time.
Immunosuppression and infection risk
Agamree or other corticosteroids suppress the immune system and increase the risk of infections. This may include:
- reduced resistance to new infection
- worsening of existing infections
- increased risk of infection spread
- increased risk of reactivating or exacerbating a latent (inactive) infection
- masked signs of infection.
Corticosteroid-associated infections may be severe, and at times fatal. The risk of infectious complications increases with higher corticosteroid doses. Patients should be monitored for the development of infections and the dose of Agamree should be reduced or withdrawn as needed.
Heart and kidney dysfunction
The chronic use of corticosteroids, including Agamree, can cause high blood pressure, salt and water retention, and increased potassium and calcium excretion. Patients should be monitored for blood pressure changes and potassium levels.
Agamree should be used with caution by patients with congestive heart failure, high blood pressure, or poor kidney function. Data indicate a risk of a rupture in the heart’s wall among those who have recently had a heart attack, thus Agamree should be used with great caution in those patients.
Gastrointestinal damage
Corticosteroids raise the risk of tears in the gastrointestinal lining (perforations) among people with certain existing gastrointestinal disorders, such as ulcers, diverticulitis, fresh intestinal anastomoses, or ulcerative colitis. Agamree should be avoided if there is a likelihood of these conditions occurring.
Symptoms of a perforation, such as redness or swelling in the lining of the abdomen (peritoneal irritation) may be masked in patients using corticosteroids.
Behavioral and Mood Disturbances
Agamree and other corticosteroids may lead to psychiatric adverse reactions that are in some cases severe. Symptoms typically emerge in the first days or weeks after starting treatment and may be dose-related. Reactions usually ease with a dose reduction or cessation, but other treatment may be needed.
For adults, symptoms may include mood swings, euphoria, or insomnia during treatment, and depressive episodes after stopping treatment. Children may have signs of hyperactivity (irritability, aggression, tantrums, mood swings) and sleep disorders. Caregivers should seek medical attention if these symptoms develop, especially if a depressed mood or suicidal thoughts are suspected.
Effects on bones, growth, and development
Corticosteroids such as Agamree can inhibit bone growth in pediatric patients or lead to bone loss (osteoporosis) in patients of any age, making them more prone to bone fractures. A person’s risk of osteoporosis should be considered before starting corticosteroids, and bone health should be monitored throughout treatment with Agamree.
Long-term corticosteroid use can also have negative effects on growth and development in children.
Eye effects
Agamree may be associated with certain eye-related symptoms, including cataracts — a clouding of the eye that affects vision — infections, and glaucoma, whose risk is increased in people with high pressure in the eye. Pressure in the eye should be monitored if Agamree is used for more than six weeks. Corticosteroids are not recommended for patients with an active eye infection with the herpes simplex virus.
Vaccinations
Live or live attenuated vaccines should not be administered to people receiving immune-suppressing doses of corticosteroids, including Agamree. Such vaccines should be administered at least 4-6 weeks before starting treatment.
Myopathy
Patients receiving corticosteroids along with other inhibitors of nerve-muscle communication (transmission), or those with diseases of neuromuscular transmission such as myasthenia gravis, may be at an increased risk of muscle disease, or myopathy, which may involve the eye, breathing, or limb muscles. Clinical improvement after stopping corticosteroids may take weeks or years.
Kaposi’s sarcoma
Kaposi’s sarcoma, a type of cancer in the cells of the gastrointestinal tract, has been reported in people taking corticosteroids, usually for chronic conditions. Stopping corticosteroids may result in clinical improvement.
Blood clots
Observational studies have indicated there may be an increased risk of certain blood clots, or thromboembolic events, with higher doses of corticosteroids. Agamree should be used with caution in patients who may be predisposed to blood clots.
Allergic reactions
Rare instances of anaphylaxis, a life-threatening allergic reaction, have been reported in patients receiving corticosteroid therapy.
Pregnancy and breastfeeding
Agamree is indicated for treating DMD, which generally affects only male patients. Therefore, there are no data on its use in pregnancy. Nevertheless, corticosteroids in general should only be used during pregnancy if the potential benefits outweigh the risk to the developing fetus, which should be determined with a person’s healthcare team.
Muscular Dystrophy News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- New research reveals protein pathway that can slow muscle repair
- Dreaming of solutions to the Olympic-sized challenges of FSHD
- Roche halts development of satralizumab for DMD bone health
- An essay on choosing hope in life with a progressive, degenerative disease
- I have new criteria for when my sons participate in DMD clinical trials
Related articles
 Fact-checked by
Fact-checked by 



