FAQs about exon-skipping therapies
Certain regulatory approvals have been granted in the U.S. to four exon-skipping therapies designed to treat amenable patients with Duchenne muscular dystrophy (DMD): Exondys 51, Vyondys 53, Viltepso, and Amondys 45. These accelerated approvals were based on early clinical biomarker data showing the therapies can increase production of dystrophin protein, which implies they are likely to provide some benefits for patients. Clinical trials are ongoing to assess whether and to what extent these therapies affect the progression of DMD.
The U.S. Food and Drug Administration (FDA) has granted accelerated approval to four exon-skipping therapies for people with Duchenne muscular dystrophy (DMD) caused by specific mutations. Through the accelerated approval pathway, the FDA can authorize the sale of therapies based on early clinical biomarker data suggesting the treatment is likely to benefit patients. Clinical trials are ongoing to confirm whether and to what extent exon-skipping medications actually affect DMD disease progression.
Some insurance providers may cover exon-skipping therapies for Duchenne muscular dystrophy (DMD). The two companies that market approved exon-skipping therapies — Sarepta Therapeutics, through the SareptAssist program, and NS Pharma, through NS Support — provide information about insurance benefits and other resources for patients and caregivers. Patients are advised to consult with their specific insurance provider to understand policies.
Some exon-skipping therapies may pose a risk of kidney damage or allergic reactions. Like any medication, exon-skipping therapies also can cause adverse events; specific side effect profiles vary depending on the therapy. Patients are advised to talk to their healthcare team about the potential risks of starting a new therapy.
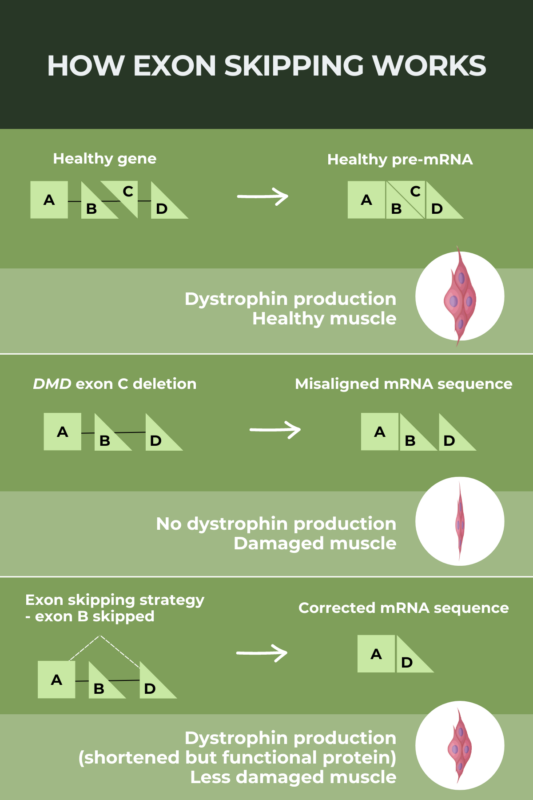
Exon skipping is a therapeutic strategy designed to allow people with Duchenne muscular dystrophy (DMD) caused by specific mutations to produce a shortened but still functional version, of the dystrophin protein they lack. People with DMD typically have no dystrophin protein at all; by allowing the production of a dystrophin protein with some functionality, exon-skipping therapies aim to slow disease progression. Four exon-skipping therapies have been approved in the U.S. based on early data showing they can increase dystrophin protein production.
 Fact-checked by
Fact-checked by