FAQs about gene therapy for Duchenne muscular dystrophy
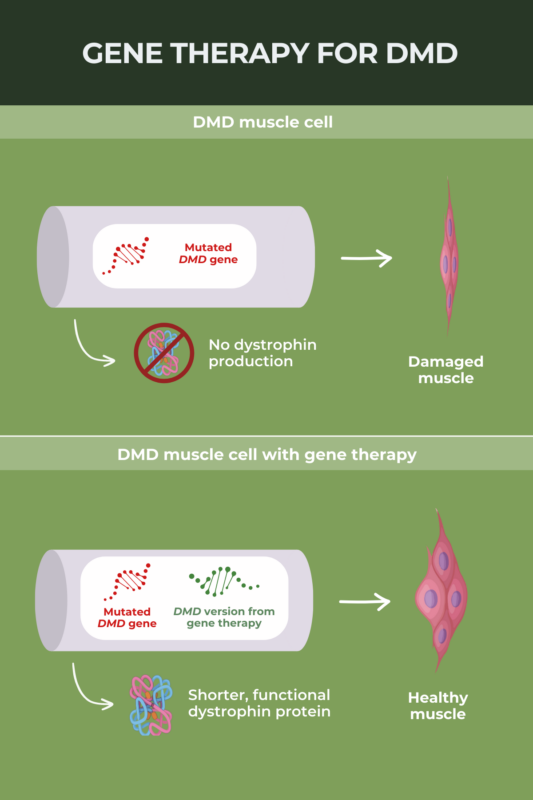
Elevidys, a gene therapy developed by Sarepta Therapeutics, was approved in the U.S. in June 2023 as a treatment for Duchenne muscular dystrophy (DMD). The therapy is designed to deliver to muscle cells a gene encoding micro-dystrophin, a shortened but functional version of the dystrophin protein that is missing in DMD. The U.S. Food and Drug Administration (FDA) granted accelerated approval to Elevidys based on early biomarker data suggesting it delivers micro-dystrophin to muscles as expected; clinical trials evaluating how the gene therapy affects disease progression are still ongoing.
Duchenne muscular dystrophy (DMD) is caused by mutations in the DMD gene that provide instructions for making the muscle protein dystrophin. The overarching aim of gene therapy for DMD is to deliver a functional version of this gene to muscle cells, allowing patients’ muscles to make a working form of dystrophin protein.
The gene therapy Elevidys was approved to treat some children with Duchenne muscular dystrophy (DMD) in the U.S. in June 2023. The therapy’s developer, Sarepta Therapeutics, priced it at $3.2 million per patient; although, according to the company, Medicaid and other programs might provide a discount, lowering the net price of Elevidys.
The U.S. Food and Drug Administration granted accelerated approval to the gene therapy Elevidys for Duchenne muscular dystrophy (DMD) based on biomarker data suggesting the treatment works as designed. Early data have suggested that children treated with the therapy tend to retain better motor function than is typically seen without treatment, but clinical trials to confirm whether and how this and other gene therapies might affect the progression of DMD are still ongoing.
Elevidys, the only gene therapy approved to date for Duchenne muscular dystrophy (DMD), can cause side effects including nausea, vomiting, fever, and low platelet counts. More serious complications, including liver damage, heart inflammation, and muscle inflammation, have been reported for Duchenne patients given gene therapy.

 Fact-checked by
Fact-checked by